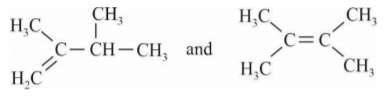
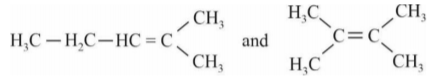
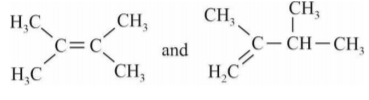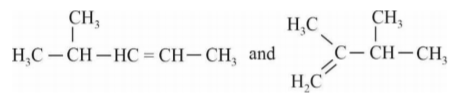A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When 3,3-Dimethyl-2-butanol is heated with acid H(3)PO(4), it is con...
Text Solution
|
- When 3,3- dimethyl -2- butanol is heated with H(2)SO(4) the major prod...
Text Solution
|
- Alcohol A (C(10)H(18)O) is converted into mixture of alkene B and C on...
Text Solution
|
- On acid hydration of how many alkene 2,3-dimethyl-2-butanol will be pr...
Text Solution
|
- When 3,3-dimethyl-2-butanol is heated with H(2)SO(4) the major product...
Text Solution
|
- What is major product formed when 3,3-dimethyl -2-butanol is heated in...
Text Solution
|
- Addition of HCl to 3,3-dimethyl-1-butene and reaction of HCl with 3,3-...
Text Solution
|
- When neopentyl alcohol is heated with an acid , it slowly converted in...
Text Solution
|
- Which one is correct statement? Basicity of \(H{3}PO{4}\)and \(H{3}PO{...
Text Solution
|