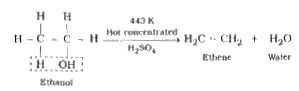Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
KUMAR PRAKASHAN|Exercise ACTIVITY|22 VideosCARBON AND ITS COMPOUNDS
KUMAR PRAKASHAN|Exercise TEXTUAL EXERCISE|14 VideosACID , BASES AND SALT
KUMAR PRAKASHAN|Exercise Activity|47 VideosCHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise PRACTICAL SKILL BASED QUESTIONS WITH ANSWERS|5 Videos
KUMAR PRAKASHAN-CARBON AND ITS COMPOUNDS-Practical Skill Based Questions With Answers
- Explain : Chemical reactions of ethanol
Text Solution
|
- An organic compound 'A' is widely used to preserve the pickles and has...
Text Solution
|
- An organic compound 'A' is widely used to preserve the pickles and has...
Text Solution
|
- An organic compound 'A' is widely used to preserve the pickles and has...
Text Solution
|
- An organic compound 'A' is widely used to preserve the pickles and has...
Text Solution
|
- Identify the compounds A, B, C, D, E and F in the following reactions ...
Text Solution
|
- Identify the compounds A, B, C, D, E and F in the following reactions ...
Text Solution
|
- Identify the compounds A, B, C, D, E and F in the following reactions ...
Text Solution
|
- Identify the compounds A, B, C, D, E and F in the following reactions ...
Text Solution
|
- Identify the compounds A, B, C, D, E and F in the following reactions ...
Text Solution
|
- An organic compound P when heated with excess concentrated H(2)SO(4) a...
Text Solution
|