A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
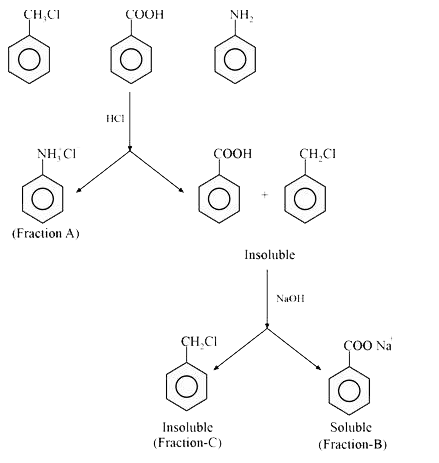
- A solution of Benzyl chloride, Benzoic acid and Aniline was extracted ...
Text Solution
|
- An analust is trying to separate a mixture containing the following fo...
Text Solution
|
- An analust is trying to separate a mixture containing the following fo...
Text Solution
|
- An analust is trying to separate a mixture containing the following fo...
Text Solution
|
- The sodium extract of an organic compound on treatment with FeSO(4) s...
Text Solution
|
- The sodium extract of an organic compound on treatement with FeSO(4) s...
Text Solution
|
- A solution of m - chloroaniline, m- chlorophenol and m - chlorobenzoin...
Text Solution
|
- A solution of Benzyl chloride, Benzoic acid and Aniline was extracted ...
Text Solution
|
- A solution of Benzylamine, Cyclohexanol and Picric acid in ethyl aceta...
Text Solution
|