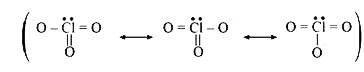
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Chlorine reacts with hot and conc. Ba (OH)(2) and produces compound X,...
Text Solution
|
- When CaC(2) reacts with N(2) a compound X is formed. X on reaction wit...
Text Solution
|
- Copper reacts with hot conc. H(2)SO(4) to give:
Text Solution
|
- A neutral organic compound X of molecualr formula C(2)H(6)O on oxidati...
Text Solution
|
- The following reaction is a/an Ba(OH)(2)+H(2)SO(4) to BaSO(4)+2H(2)O
Text Solution
|
- Cl 2 on reaction with hot & conc. NaOH gives two chlorine having ...
Text Solution
|
- A compound X declourises Br(2) water and reacts slowly with conc H(2)S...
Text Solution
|
- Cl(2)(g)+Ba(OH)(2) to X(aq.)+BaCl(2)+H(2)O X+H(2)SO(4) to Y+BaSO(4) Y ...
Text Solution
|
- What happens to KMnO(4) when it reacts with (i) cold conc. H(2) SO(4) ...
Text Solution
|