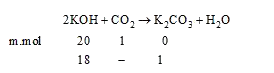A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A bottle, which contains 200 ml of 0.1 M KOH, absorbs 1 millimole of C...
Text Solution
|
- Air sample from an industrial area of Delhi, which is heavily polluted...
Text Solution
|
- 0.1g of a solution containing Na(2)CO(3) and NaHCO(3) requires 10mL of...
Text Solution
|
- A bottle of cold drink has 200 mL liquid in which CO(2) is 0.1 molar. ...
Text Solution
|
- A mixture of KOH and Na(2)CO(3) solution required 15 mL of N//20 HCl u...
Text Solution
|
- A 0.1 N solution of Na(2)CO(3) is titrated with 0.1 N HCl solution. Th...
Text Solution
|
- A solution containing Na(2)CO(3) and NaOH requires 300 mL of 0.1 N HCl...
Text Solution
|
- A bottle of cold drink has 200 mL liquid in which CO(2) is 0.1 molar. ...
Text Solution
|
- A bottle, which contains 200 ml of 0.1 M KOH, absorbs 1 millimole of C...
Text Solution
|