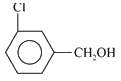
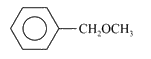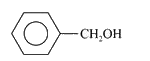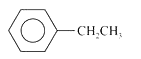A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compound is most reactive with HCl in presence ...
Text Solution
|
- Which alcohol is most reactive towards HCl in the presence of anhydrou...
Text Solution
|
- Assertion : Lucas reagent is a mixture of conc. HCl and anhydrous ZnCl...
Text Solution
|
- ZnCl2 in Lucas reagent is
Text Solution
|
- Which of the following is the most reactive with HCl in the presence o...
Text Solution
|
- Which of the following will react most easily with HCl in the presence...
Text Solution
|
- When alcohol are reacted by HCl,in presence of anhydrous ZnCl2 , the l...
Text Solution
|
- Which of the following alcohols is most reactive with HCl in the prese...
Text Solution
|
- प्रक्कथन : ल्यूकास अभिकर्मक निर्जल ZnCl2 एवं सान्द्र HCl का मिश्रण है।...
Text Solution
|