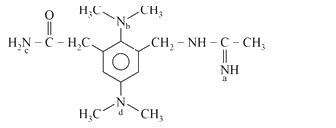
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The increasing order of pK(b) in the following compound is:
Text Solution
|
- Arrange the following compounds in their increasing pK(b) order: (a). ...
Text Solution
|
- Correct Decreasing order of pK(b) value of Following Compound is
Text Solution
|
- Arrange above phenol in increasing order of pK(a) value:
Text Solution
|
- The increasing order of pK(a) values of the following compounds is :
Text Solution
|
- The increasing order of the pK(b) of the following compound is:
Text Solution
|
- The increasing order of pK(b) for the following amines is
Text Solution
|
- The increasing order of PK(b) for the following compounds will be (1...
Text Solution
|
- The increasing order of the pK(a) value of the following compounds is
Text Solution
|