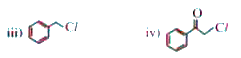A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 A (CHLOROFORM)|3 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 A (MECHANISM OF NUCLEOPHILLIC SUBSTITUTION REACTIONS)|24 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 A (INTRODUCTION, NATURE OF C-X BOND)|20 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 6 (Integer answer type Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALKYL AND ARYL HALIDES-OBJECTIVE EXERCISE - 2 A (PREPARATION AND PROPERTIES OF ETHYL CHLORIDE)
- Ethyl chloride on heating with silver cyanide forms a compound X. The ...
Text Solution
|
- Hydrogen chloride and So, are the by products in the reaction of ethan...
Text Solution
|
- Covalence of carbon in the functional group of C and D are
Text Solution
|
- C2H5Cl("excess") overset(NH3 alc)(rarr)A("final")Covalence of 'N' in '...
Text Solution
|
- Which one of the following reaction is Swart reaction ?
Text Solution
|
- CH3COOAg + C2H5Cl rarr A (organise) Wrong statement about 'A' is
Text Solution
|
- Ethyl chloride can be converted into ethane by reacting with
Text Solution
|
- CH3CH2Cl underset(Delta)overset(alc. KOH)(rarr) X((org)) Wrong state...
Text Solution
|
- Identify Z in the following series C2H5I overset(Alc. KOH)(rarr) X ove...
Text Solution
|
- Compound A reacts with PCI5 to give B which on treatment with KCN foll...
Text Solution
|
- The carbon compound "A" forms "B" with sodium metal and again forms "C...
Text Solution
|
- 1-Bromopropane on reaction with LiAlH4 yields
Text Solution
|
- CH3-CH2-CH2-Cl underset(KOH)overset(alc)(rarr) B overset(HBr)C underse...
Text Solution
|
- Ethyl bromide reacts with lead-sodium alloy to form
Text Solution
|
- (i) CH3CH2 Cl overset("KOH"("aqueous"))to X (ii) underset( 170^(@...
Text Solution
|
- What are X and Y respectively in the following reaction? X overset(P...
Text Solution
|
- C2H5Cl + Mg rarr x overset(H2O)(rarr) Y. C2H5Cl overset(LiAlH4)(rarr) ...
Text Solution
|
- What are the reagent and reaction conditions used for converting ethyl...
Text Solution
|
- In the following sequence of reactions CH3Br overset(KCN)(rarr)A ove...
Text Solution
|
- The reactivity order of below compounds with KI in acetone is : i) C...
Text Solution
|