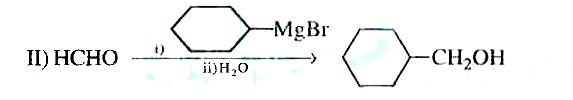Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise EXERCISE - 2.1.1|9 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise EXERCISE - 2.1.2|8 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise ETHERS(Practice exercise)|13 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALCOHOLS, PHENOLS AND ETHERS-EXAMPLES
- Give the structures and IUPAC names of the products expected from the ...
Text Solution
|
- Write structures of prudlucts of the following reactions : I) CH3CH=...
Text Solution
|
- Show how are the following alcohols are prepared by the reaction of a ...
Text Solution
|
- Arrange the following sets of compounds in order of their increasing b...
Text Solution
|
- Write the aromatic structures of all isomers with the formula C7H8O.
Text Solution
|
- What is the tautomer of phenol ? Which is more stable ?
Text Solution
|
- How is salicylic acid converted to phenol?
Text Solution
|
- Explain the method of preparation of resorcinol from benzene.
Text Solution
|
- Phenol decolourises reddish brown colour of bromine, though it is not ...
Text Solution
|
- Write the decreasing order of acidic strengths of the following: A :...
Text Solution
|
- Write the electrophile and intermediate in Reimer-Tiemann reaction.
Text Solution
|
- What is the reason for the formation of tribromo phenol when phenol is...
Text Solution
|
- Phenol is treated with C Cl(4) in presence of NaOH. What happens ?
Text Solution
|
- Write the structures of the major products expected from the following...
Text Solution
|
- Dehydration of alcohols is suitable for preparing ethers having primar...
Text Solution
|
- Give one example of an ether which cannot be decomposed by HI.
Text Solution
|
- Ether can be dried over sodium, but not alcohol. Why?
Text Solution
|
- Chloroethane, 2-chloropropane and chloroethene are treated with methox...
Text Solution
|
- Diphenyl ether cannot cleaved by treatment HI. Why?
Text Solution
|
- How is ether distinguished from ethanol using iodine and alkali ?
Text Solution
|