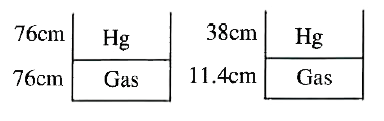Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A vertical hollow cylinder of height 1.52 m is fitted with a movable p...
Text Solution
|
- A vertical cylinder of height 100cm contains air at a constant tempera...
Text Solution
|
- A glass cylinder contains m0=100g of mercury at a temperature of t0=0^...
Text Solution
|
- A vertical hollow cylinder of height 1.52m is fitted with a movable pi...
Text Solution
|
- A vertical hollow cyclinder of height 1.52 m is fitted with a movable ...
Text Solution
|
- A vertical cylinder of height 1.52m is fitted with a movable piston of...
Text Solution
|
- A cylinder of iron floats vertically and fully immersed in a vessel c...
Text Solution
|
- A flat uniform cylinder of lead floats in mercury at 0^(@)C. Will the ...
Text Solution
|
- A gas is filled in a cylinder fitted with a piston at a definite tempe...
Text Solution
|