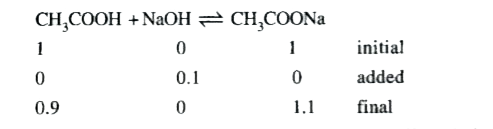Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- One litre of buffer solution contains 0.1 mole of acetic acid add 1 mo...
Text Solution
|
- A buffer solution contains 0.1 M of acetic acid and 0.1 M of sodium a...
Text Solution
|
- A buffer solution contains 0.1 mole of sodium acetate dissolved in 100...
Text Solution
|
- A buffer solution contains 0.1 mole of sodium acetate in 1000 cm^(3) o...
Text Solution
|
- 50 ml o 0.1 M sodium acetate, 25 ml of 0.2 M acetic acid were added to...
Text Solution
|
- One litre of buffer solution contains 0.1 mole of acetic acid add 1 mo...
Text Solution
|
- A buffer solution contains 0.1 mole of sodium acetate dissolved in 100...
Text Solution
|
- The pH of a buffer solution containing equal moles of acetic acid and ...
Text Solution
|
- A buffer solution contains 0.1 mole of sodium acetate in1000 cm^(3) of...
Text Solution
|