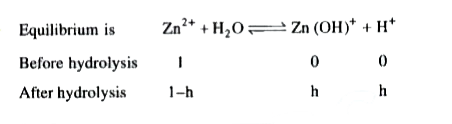Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the pH of 0.001 M aqueous solution of ZnCl2, Ka for the equi...
Text Solution
|
- The salt ZN (OH)(2) is involved in the following two equilibria: Zn(OH...
Text Solution
|
- ZnCl(2) undeoes hydrolysis ZnCl(2) + H(2)O hArr Zn(OH)(2) + 2HCl. The ...
Text Solution
|
- Calculate the emf of the following cell at 25^(@)C . Zn|Zn^(2+) (0.001...
Text Solution
|
- The degree of hydrolysis in hydrolic equilibrium A^(-) + H(2)O hArr HA...
Text Solution
|
- The equilibrium A^(-) + H(2)O hArr HA + OH^(-) (K(a) = 1.0 xx 10^(-5))...
Text Solution
|
- The acid ionization (hydrolysis) constant of Zn^(2+)" is "1.0 xx 10^(-...
Text Solution
|
- The acid ionization constant for Zn^(2+)+H(2)O hArr (OH^(+)) + H^(+) i...
Text Solution
|
- The acid ionization (hydrolysis) constant of Zn^(2+)" is "1.0 xx 10^(-...
Text Solution
|