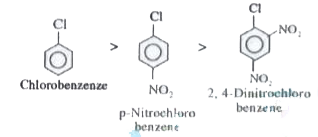
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following set of compounds in order of their decreasing re...
Text Solution
|
- Arrange the following set of compounds in order of their decreasing re...
Text Solution
|
- Arrange the following set of compounds in order of their decreasing re...
Text Solution
|
- निम्नलिखित यौगिकों को उनकी इलेक्ट्रॉनस्नेही (E^(+)) के प्रति घटती आपेक...
Text Solution
|
- Arrange the following compounds in order of their decreasing reactivit...
Text Solution
|
- Arrange the following set of compounds in order of their decreasing re...
Text Solution
|
- Arrange the following set of compounds in order of their decreasing re...
Text Solution
|
- Arrange the following set of compounds in order of their decreasing r...
Text Solution
|
- Arrange the following set of compounds in the order of their decreasin...
Text Solution
|