Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 2 (CARCINOGENICITY)|5 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 1 (CARCINOGENICITY)|3 VideosALKALINE EARTH METALS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE ADDITIONAL QUESTIONS|15 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AROMATIC HYDROCARBONS-OBJECTIVE EXERCIES - 2 (BENZENE)
- Among the following compounds, which are not aromatic ?
Text Solution
|
- Deactivating nature and o- ,p- directing nature of halogens can be exp...
Text Solution
|
- CaC(2) + 2H(2)Orarr X CH3 - CH(2) Cl overset("alc" KOH) rarr Y, X o...
Text Solution
|
- C6H6 easily shown
Text Solution
|
- LIST - 1 (Conversion) A) Benzene to Cyclohexane B) Ethylene to For...
Text Solution
|
- Ozonolysis of one mole of benzene gives
Text Solution
|
- Ethyl benzene with Br2 in the presence of FeBr3 gives
Text Solution
|
- Cyclohexene adds one mole of H2, where the enthalpy of hydrogenation i...
Text Solution
|
- Regarding Benzene some statements are given A) It is aromatic B) I...
Text Solution
|
- Which of the following is not an aromatic compound
Text Solution
|
- Sodium benzoate gives Benzene on being heated with 'X'. Phenol gives B...
Text Solution
|
- The major product in the reaction of benzene with n-propyl chloride in...
Text Solution
|
- Which of the following species is aromatic?
Text Solution
|
- Characterisitc reactions of benzene are A) addition reactions B) s...
Text Solution
|
- Although benzene is highly unsaturated it does not undergo addition re...
Text Solution
|
- Benzene on ozonolysis followed by reaction with Zn and H2O gives 3 mol...
Text Solution
|
- Which one of the following statements is correct?
Text Solution
|
- A miscible mixture of C6H6 + CHCl3 can be separated by
Text Solution
|
- Electrophile that participates in nitration of benzene is
Text Solution
|
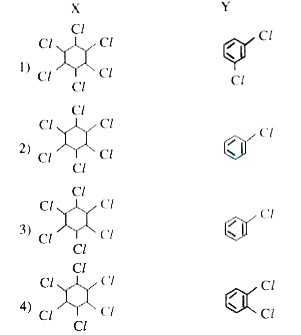
- Identify X and Y in the following reactions
Text Solution
|