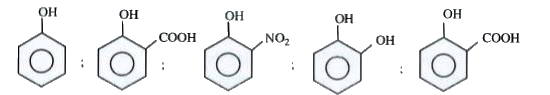
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise PRACTICE SHEET - EXERCISE-I (LEVEL-I Straight Objective Type Questions)|10 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise PRACTICE SHEET - EXERCISE-I (LEVEL-II Straight Objective Type Questions)|7 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise LECTURE SHEET - EXERCISE-III (Matrix Matching Type Questions)|1 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-LECTURE SHEET - EXERCISE-III (Integer Type Questions)
- How many of the following acids are more acidic than benzoic acid?
Text Solution
|
- Number of compounds having relatively more acidic hydrogen than Me(3)C...
Text Solution
|
- The number of acids which are more acidic than benzoic acid.
Text Solution
|
- Which numbered nitrogen atom gets protonated first at relatively highe...
Text Solution
|
- How many of the following compounds are more basic than ?
Text Solution
|
- How many of the following compounds are soluble in aqueous solutions o...
Text Solution
|
- How many of the following species have dipolement?
Text Solution
|
- How many of the following statements are correct? i) In cyclobutadie...
Text Solution
|
- In the following aromatic, anti-aromatic and non-aromatic compounds ar...
Text Solution
|
- The number of acids which are more acidic than water. CH(3)-OH, C(2)...
Text Solution
|
- How many statements are correct of the following compounds I is m...
Text Solution
|