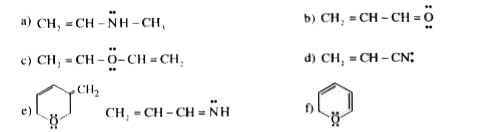Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (Practive Sheet (ADVANCED) Straight Objective Type Questions)|38 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (Practive Sheet (ADVANCED) Linked Comprehension Type Questions)|3 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) Linked Comprehension Type Questions)|3 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) Integer Type Questions)
- How many of the following statements is (are) true about resonance. ...
Text Solution
|
- How many of the following lone-pair indicated is involved in resonance...
Text Solution
|
- How many of the following lone-pair indicated is not involved in reson...
Text Solution
|
- Find the number of correct statement(s): a) CH(3)Ooverset(oplus)(C)H...
Text Solution
|