A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (Practive Sheet (ADVANCED) Linked Comprehension Type Questions)|3 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (Practive Sheet (ADVANCED) Integer Type Questions)|4 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II LECTURE SHEET (ADVANCED) Integer Type Questions)|4 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3|7 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELECTRONIC EFFECTS AND REACTION INTERMEDIATES-ADDITIONAL PRACTICE EXERCISE (Practive Sheet (ADVANCED) Straight Objective Type Questions)
- Which of the following represent the given mode of hybridisation sp2, ...
Text Solution
|
- Maximum dipole moment will be of :
Text Solution
|
- Correct order of acidic strength is:
Text Solution
|
- For 1-methoxy-1,3-butadiene, which of the following resonating structu...
Text Solution
|
- When benzene sulfonic acid and p-nitrophenol are treated with NaHCO(3)...
Text Solution
|
- Among the following, the least stable resonance structure is
Text Solution
|
- The correct stability order of the following species is
Text Solution
|
- Hyper conjugation involves overlap of the following orbitals
Text Solution
|
- The correct acidity order of the following is :
Text Solution
|
- The correct stability order of the following resonance structure is : ...
Text Solution
|
- Among the following compounds, the most acidic is
Text Solution
|
- Which of the following statements would be false about this compound :
Text Solution
|
- Which of the following statement is/are correct?
Text Solution
|
- Which of the following compounds reacts with NaHCO(3)" giving "CO(2)
Text Solution
|
- In which pair IInd compound is weak acids.
Text Solution
|
- Among the following which statement(s) is/are true:
Text Solution
|
- In which of the following pair IInd compound is weaker base.
Text Solution
|
- Amongst the following statements, which is(are) correct ?
Text Solution
|
- Among the following which one is having conjugated system :
Text Solution
|
- Choose the correct statement :
Text Solution
|
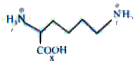 Correct order of acidic strength is:
Correct order of acidic strength is: