A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 3 (VERY SHORT ANSWER QUESTIONS)|13 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMIC STRUCTURE-OBJECTIVE EXERCISE - 2
- The energy of an electromagnetic radiation is 3 xx 10^(-12) ergs. What...
Text Solution
|
- If a metal is irradiated with light of frequency 3 xx 10^(19) sec^(-1)...
Text Solution
|
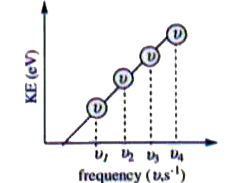
- In a photoelectric experiment, kinetic energy of photoelectrons was pl...
Text Solution
|
- Calculate the mass of a photon with wavelength 3.6 Å.
Text Solution
|
- The wavelength of the first member of the Balmer series in hydrogen s...
Text Solution
|
- Which of the following transitions will have minimum wavelength ?
Text Solution
|
- The ratio of wavelength values of series limit lines (n(2) =oo) of Bal...
Text Solution
|
- What electronic transition in Li^(2+) produces the radiation of the sa...
Text Solution
|
- The ratio of the wave lengths of the first line in the Lyman series of...
Text Solution
|
- The number of spectral lines that are possible when electrons in 7^("t...
Text Solution
|
- In a certain electronic transition from the quantum level, 'n' to the ...
Text Solution
|
- The minimum and maximum values of wave length in the Lyman series of a...
Text Solution
|
- Which of the following transitions of an electron in hydrogen atom emi...
Text Solution
|
- In a hydrogen atom, the electron is at a distance of 4.76Å from the nu...
Text Solution
|
- Which of the following statements regarding spectral series is correct...
Text Solution
|
- In a series in the line spectrum of hydrogen the wavelength of radiati...
Text Solution
|
- The frequency of the spectral line obtained when the electron in n = 3...
Text Solution
|
- When a greater number of electrons from excited hydrogen atoms reach t...
Text Solution
|
- The first spectral line in the Pfund series of Hydrogen spectrum is gi...
Text Solution
|
- A certain transition in H spectrum from an excited state to ground sta...
Text Solution
|