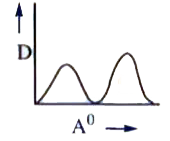
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 3 (VERY SHORT ANSWER QUESTIONS)|13 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMIC STRUCTURE-OBJECTIVE EXERCISE - 2
- If the uncertainty in velocity of a particle of mass 0.1 kg is 6.626 x...
Text Solution
|
- There is no difference between a 2p and a 3p orbital regarding
Text Solution
|
- The set of quantum numbers 'n' and 'l' possible for the orbital shown ...
Text Solution
|
- The probability of finding electron in XY plane for P(z) - orbital is
Text Solution
|
- What are the values of the orbital angular momentum of an electron in ...
Text Solution
|
- The maximum number of electrons that can be filled into all the orbita...
Text Solution
|
- The number of sub levles in the quantum level n = 3 is
Text Solution
|
- The number of different spatial arrangements for the orbital with 1 = ...
Text Solution
|
- The maximum number of electrons that can be present in an orbit with s...
Text Solution
|
- The maximum number of electrons with S = +1//2 in an orbital for which...
Text Solution
|
- If the value of principal quantum number is 3, the total possible valu...
Text Solution
|
- An electron has magnetic quantum number as '-3'. Its principal quantum...
Text Solution
|
- In a multi- electron atom, which of the following orbitals described b...
Text Solution
|
- How many electrons maximum can have n + 1 = 4 in an atom.
Text Solution
|
- If magnetic quantum number of a given electron is represented -3, then...
Text Solution
|
- The radial probability distribution curve obtained for an orbital wave...
Text Solution
|
- The number of waves made by an electron moving in an orbit having a ma...
Text Solution
|
- Which combination of quantum numbers n, 1, m, s for the electron in an...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of Cl ato...
Text Solution
|
- Which one of the following pairs of atoms/ ions have identical ground ...
Text Solution
|