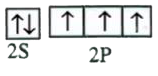
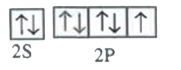
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|20 VideosATOMIC STRUCTURE
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 3 (VERY SHORT ANSWER QUESTIONS)|13 VideosAROMATIC HYDROCARBONS
AAKASH SERIES|Exercise OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)|10 VideosCHEMICAL BONDING
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )|39 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMIC STRUCTURE-OBJECTIVE EXERCISE - 2
- Which is the first element to have 4d-electron in its electronic confi...
Text Solution
|
- In the ground state, an element has 13 electrons in its "M - shell". T...
Text Solution
|
- In which of the following Aufbau principle is violated?
Text Solution
|
- Which of the following electronic configuration is not possible?
Text Solution
|
- The configuration 1s^(2)2s^(2)2p^(5)3s^(1) shows
Text Solution
|
- Which of the following has maximum unpaired d-electrons?
Text Solution
|
- Given this set of quantum numbers for a multi electron atom 2, 0, 0, a...
Text Solution
|
- Which of the following isolated gaseous atoms has highest net electron...
Text Solution
|
- If the Nitrogen atom had electronic configura - tion 1s^(7), it would ...
Text Solution
|
- Which one of the following sets of quantum numbers is not possible for...
Text Solution
|
- According to aufbau principle, the correct order of energy of 3d, 4s a...
Text Solution
|
- The electrons identified by quantum numbers n and I (i) n = 4,l = 1 ...
Text Solution
|
- Which of the following sets of quantum numbers is correct for an elect...
Text Solution
|
- Which one of the following represents an impossible arrangement of ele...
Text Solution
|
- If n = 6, the correct sequence for filling of electrons will be
Text Solution
|
- The number of unpaired electrons present in palladium (Z = 46) atom is
Text Solution
|
- The incorrect electronic arrangment is
Text Solution
|
- Which of the following has non-spherical sub shell of electron ?
Text Solution
|
- Which of the following is violation of Pauli's exclusion principle ?
Text Solution
|
- What are the total number of orbitals and electrons for m = 0, if ther...
Text Solution
|