Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC NITROGEN COMPOUNDS
NCERT TELUGU|Exercise Diazonium chloride|4 VideosORGANIC NITROGEN COMPOUNDS
NCERT TELUGU|Exercise SELF EVALUATION((C ) Answer not exceeding sixty words:)|14 VideosHALOALKANES AND HALOARENES
NCERT TELUGU|Exercise Exercises|21 VideosPOLYMERS
NCERT TELUGU|Exercise Exercises|20 Videos
Similar Questions
Explore conceptually related problems
NCERT TELUGU-ORGANIC NITROGEN COMPOUNDS-SELF EVALUATION((D) Solve the problems:)
- Nitrobenzene does not undergo Friedel-Crafts alkylation. Give reasons.
Text Solution
|
- Boiling points of nitroalkanes are much higher than those of hydrocarb...
Text Solution
|
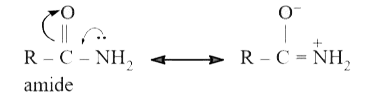
- Explain why amines are more basic than amides.
Text Solution
|
- An organic compound (A) with molecular formula C(6)H(7)N gives (B) wit...
Text Solution
|
- Outline the mechanism of (a) Nitration of aniline (b) Acetylation ...
Text Solution
|
- Outline the preparation of (a) para nitroaniline from aniline (b) ...
Text Solution
|
- Indicate the mechanism of (a) the formation of N-methyl aniline from...
Text Solution
|
- Explain the following order of strength of bases. (a) (CH(3))(2)NH g...
Text Solution
|