A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CALORIMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (LEVEL - II Matrix Matching Type Questions)|2 VideosCALORIMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (LEVEL - II Integer Type Qustions Type Questions)|4 VideosCALORIMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (LEVEL - II More than One correct Type Questions)|5 VideosAPPENDICES
AAKASH SERIES|Exercise GRAVITATION (EARTH SATELLITES AND ESCAPE SPEED)|35 VideosCENTRE OF MASS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET (ADVANCED))|3 Videos
AAKASH SERIES-CALORIMETRY -PRACTICE SHEET (LEVEL - II Linked Comprehension Type Questions)
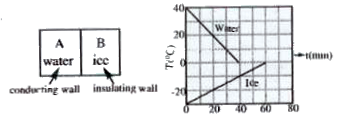
- A 0.60 kg sample of water ans a sample of ice are placed in two compar...
Text Solution
|
- A 0.60 kg sample of water ans a sample of ice are placed in two compar...
Text Solution
|
- A 0.60 kg sample of water ans a sample of ice are placed in two compar...
Text Solution
|
- A calorimeter of mass m contains an equal mass of water in it. The tem...
Text Solution
|
- A calorimeter of mass m contains an equal mass of water in it. The tem...
Text Solution
|
- A calorimeter of mass m contains an equal mass of water in it. The tem...
Text Solution
|
- A heating curve for 1 g ice at -30^@C is converted tos team, at 120°C...
Text Solution
|
- A heating curve for 1 g ice at -30^@C is converted tos team, at 120°C...
Text Solution
|
- A heating curve for 1 g ice at -30^@C is converted tos team, at 120°C...
Text Solution
|