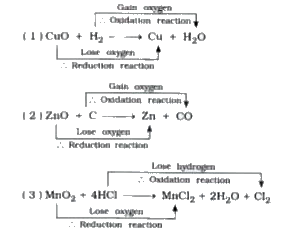
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise QUESTIONS AND ANSWERS Intext Questions (Corrosion)|1 VideosCHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise QUESTIONS AND ANSWERS Intext Questions ( Rancidity)|1 VideosCHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise QUESTIONS AND ANSWERS Intext Questions (Double Displacement reaction)|2 VideosCARBON AND ITS COMPOUNDS
KUMAR PRAKASHAN|Exercise Practical Skill Based Questions With Answers|10 VideosMETALS AND NON-METALS
KUMAR PRAKASHAN|Exercise PRACTICE SKILL BASED QUESTIONS WITH ANSWERS|5 Videos
Similar Questions
Explore conceptually related problems