Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise QUESTIONS AND ANSWERS (ACTIVITY) 1.5|3 VideosCHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise QUESTIONS AND ANSWERS (ACTIVITY) 1.6|2 VideosCHEMICAL REACTIONS AND EQUATIONS
KUMAR PRAKASHAN|Exercise QUESTIONS AND ANSWERS (ACTIVITY 1.3)|4 VideosCARBON AND ITS COMPOUNDS
KUMAR PRAKASHAN|Exercise Practical Skill Based Questions With Answers|10 VideosMETALS AND NON-METALS
KUMAR PRAKASHAN|Exercise PRACTICE SKILL BASED QUESTIONS WITH ANSWERS|5 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-CHEMICAL REACTIONS AND EQUATIONS -QUESTIONS AND ANSWERS (ACTIVITY 1.4)
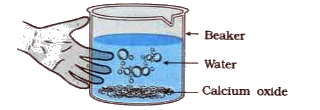
- Aim: To study the reaction between calcium oxide and water. Activity...
Text Solution
|
- Aim: To study the reaction between calcium oxide and water. Activity...
Text Solution
|
- Aim: To study the reaction between calcium oxide and water. Activity...
Text Solution
|
- Aim: To study the reaction between calcium oxide and water. Activity...
Text Solution
|