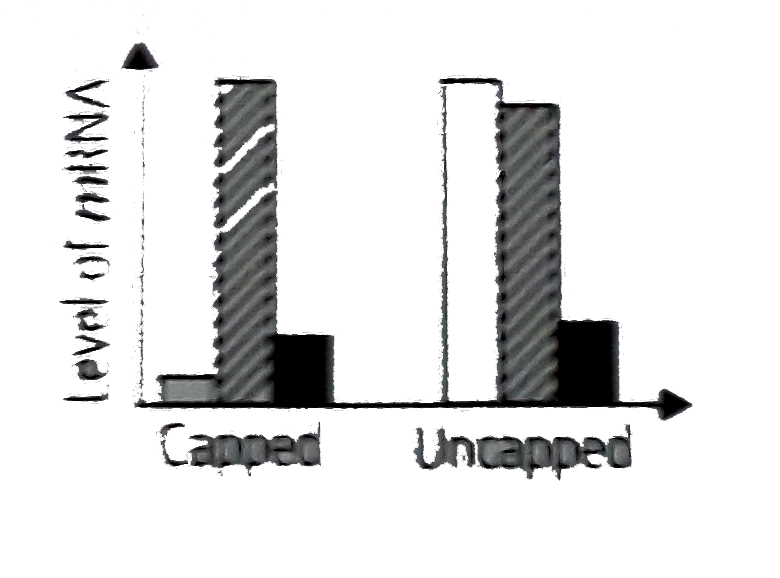A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
MOLECULAR BASIS OF INHERITANCE
NCERT FINGERTIPS ENGLISH|Exercise NCERT|28 VideosMOLECULAR BASIS OF INHERITANCE
NCERT FINGERTIPS ENGLISH|Exercise CORNER|15 VideosMOLECULAR BASIS OF INHERITANCE
NCERT FINGERTIPS ENGLISH|Exercise Molecular Basis Of Inheritance|151 VideosMICROBES IN HUMAN WELFARE
NCERT FINGERTIPS ENGLISH|Exercise Microbes In Human Welfare|138 VideosORGANISMS AND POPULATION
NCERT FINGERTIPS ENGLISH|Exercise Organisms And Population|175 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-MOLECULAR BASIS OF INHERITANCE-HOTS
- A scientist was studying an in vitro euckaryotic transcription system...
Text Solution
|
- A DNA template plus primer with the structure (where =a phosphate grou...
Text Solution
|
- C-value is the characteristic DNA content in a haploid cell of a given...
Text Solution
|
- The DNA fingerprinting analysis analysis of four family members is sh...
Text Solution
|
- An organism uses 20 amino acids while its DNA is made up of 6 types of...
Text Solution
|
- The semi-conservative nature of DNA replication was established by Me...
Text Solution
|
- You have created a fusion between trp operon and lac operon which enco...
Text Solution
|
- A DNA sequence undergoes three subsequent point mutations which result...
Text Solution
|