A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PRACTICE PAPPER
NCERT FINGERTIPS ENGLISH|Exercise Practice Papper 3|50 VideosPRACTICE PAPPER
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 1|50 VideosPRACTICE PAPPER
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|50 VideosNUCLEI
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosRAY OPTICS AND OPTICAL INSTRUMENTS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|11 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPPER-Practice Papper 2
- A current of 3 A flows through the 2 Omega resistor as shown in the ci...
Text Solution
|
- What is orbital angular momentum of an electron in 3d orbital.
Text Solution
|
- Two identical magnetic dipoles of magnetic moments 1*0Am^2 each are pl...
Text Solution
|
- Current flows through uniform square frames as shown. In which case is...
Text Solution
|
- The conducting circular loops of radii R(1) and R(2) are placed in the...
Text Solution
|
- The intensity of the light coming from one of the slits in a Young's d...
Text Solution
|
- Let v(1) be the frequency of series limit of Lyman series, v(2) the fr...
Text Solution
|
- The focal length of a biconvex lens of refractive index 1.5 is 0.06m. ...
Text Solution
|
- A metallic surface is irradiated by a monochromatic light of frequency...
Text Solution
|
- What is the energy stored in the capacitor between terminals a and b o...
Text Solution
|
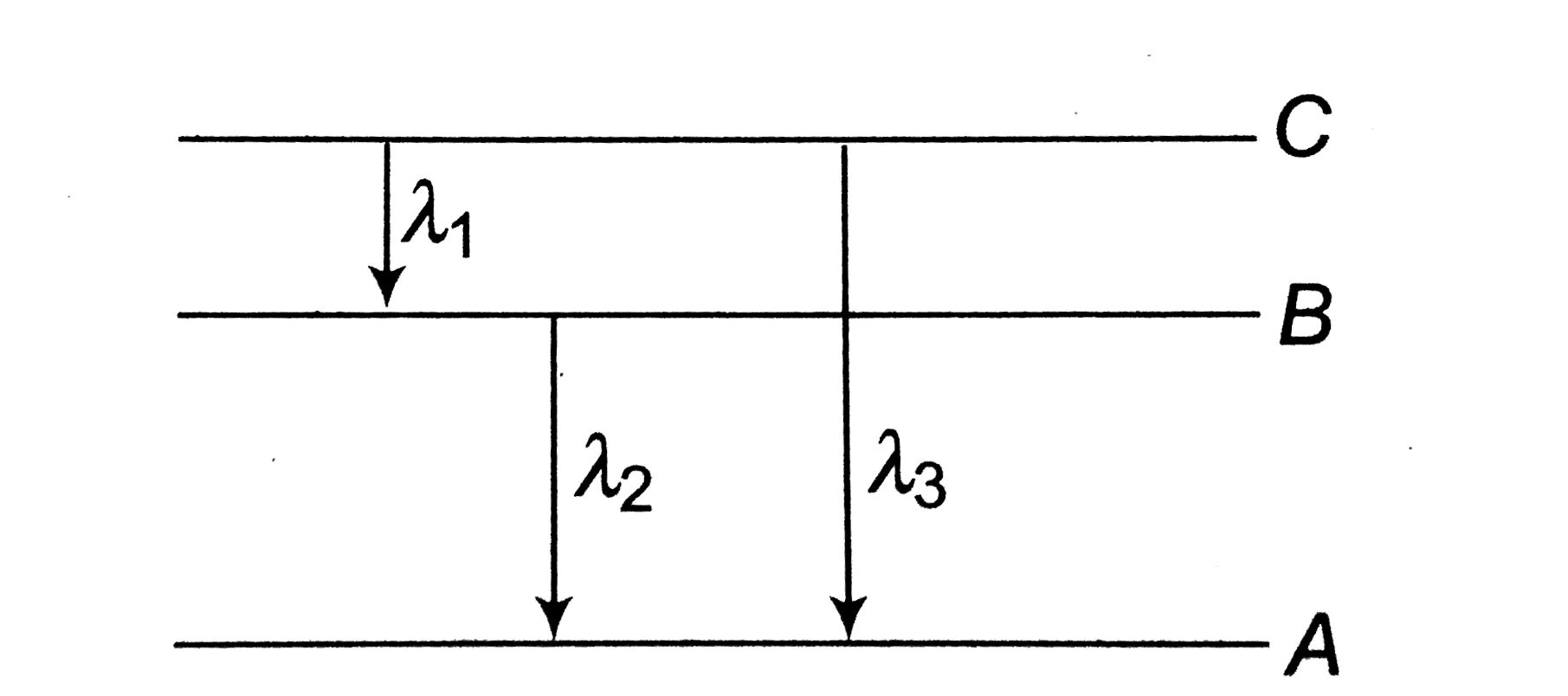
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- The real time variation of input signals A and B are as shown below. I...
Text Solution
|
- A particle of mass m and charge Q is placed in an electric filed W whi...
Text Solution
|
- In common emitter amplifier, the current gain is 62. The collector res...
Text Solution
|
- The Boolean expression for the given circuit is
Text Solution
|
- In the figure shown i=10e^(-4t)A. Find VL and V(ab)
Text Solution
|
- The total energy of a hydrogen atom in its ground state is -13.6 eV. I...
Text Solution
|
- An ac source is of (200)/(sqrt(2)) V, 50 Hz. The value of voltage afte...
Text Solution
|
- The diode used in the circuit shown in the figure has a constant volta...
Text Solution
|
- The half-life of a radioactive isotope X is 50 yr. It decays to an oth...
Text Solution
|