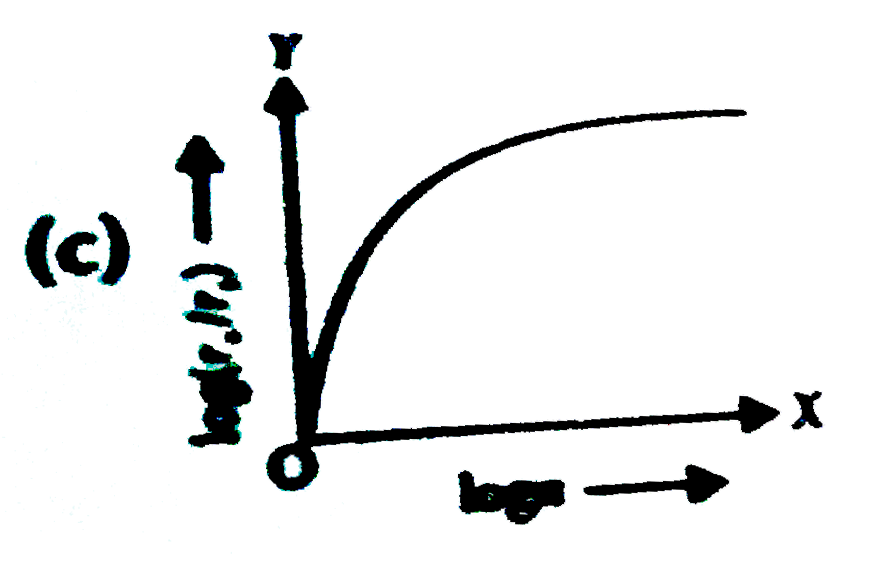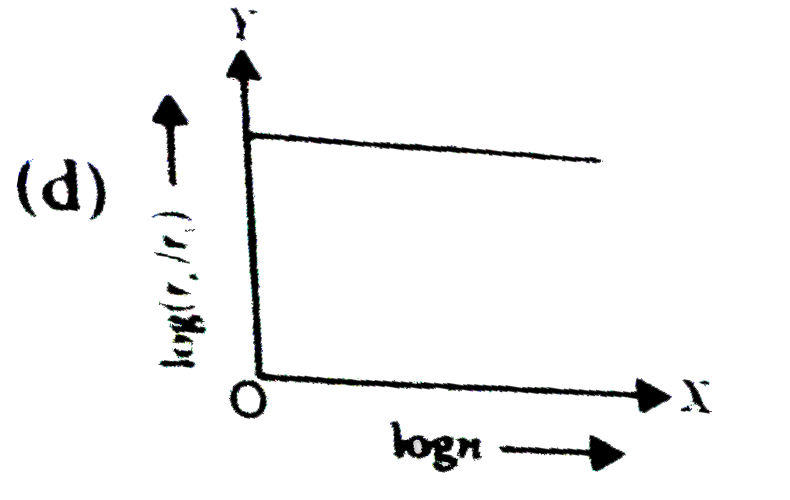A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ATOMS
NCERT FINGERTIPS ENGLISH|Exercise Higher order thinking skills|8 VideosATOMS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Examplar problems|7 VideosALTERNATING CURRENT
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosCOMMUNITCATION SYSTEMS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|30 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ATOMS -Assertion And Reason
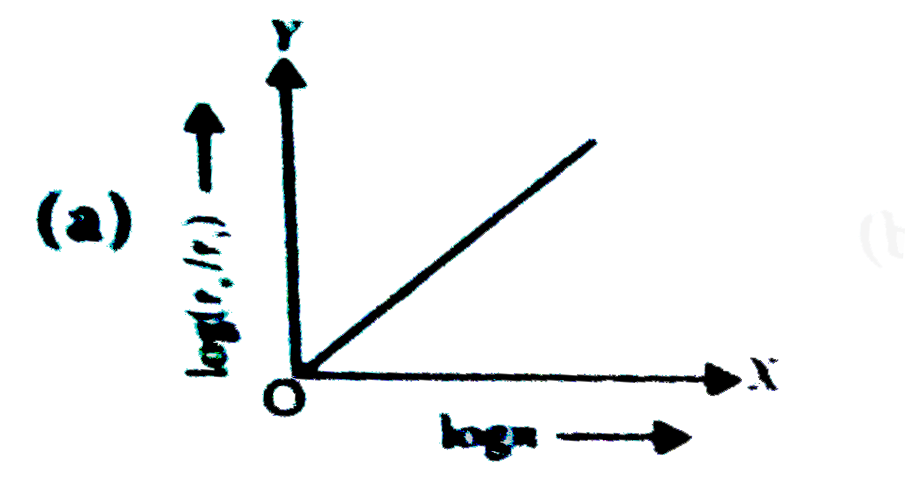
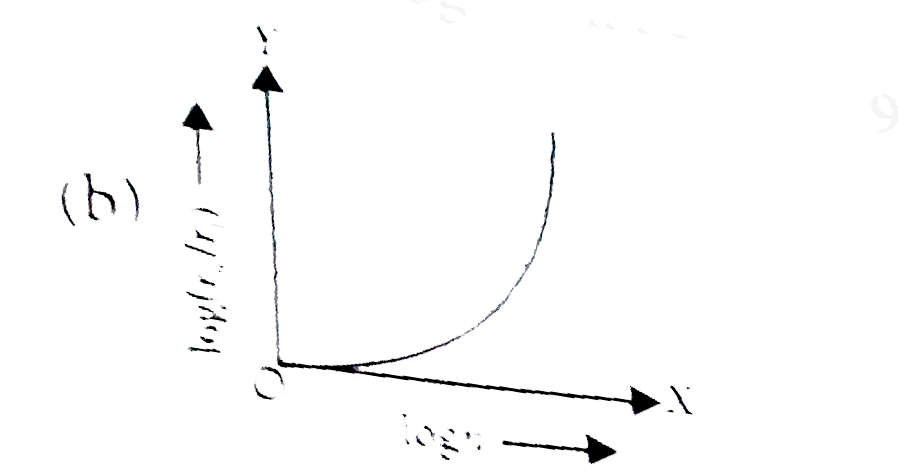
- In a hydrogen atom, the radius of n^(th) bohr orbit is rn. The graph b...
Text Solution
|
- (A) atoms of each element are stable and emit characteristic spectrum....
Text Solution
|
- (A) atom as a whole is electrically neutral. (R)atom contains equal ...
Text Solution
|
- (A) according to classical electromagnetic theory an accelerated parti...
Text Solution
|
- (A) in alpha particle scattering number of alpha paritcle undergoing h...
Text Solution
|
- (A) most of the mass of the atom is concentrated in its nucleus. (R)...
Text Solution
|
- (A) the trajetory traced by an incident particle depends on the impact...
Text Solution
|
- (A) in the experiment of alpha particle scattering, extremely thin gol...
Text Solution
|
- (A) the total energy of an electron revolving in any stationary orbit ...
Text Solution
|
- Statement -1 : Large angle scattering of alpha particles led to the di...
Text Solution
|
- Assertion: For the scattering of alpha-particles at a large angles, on...
Text Solution
|
- Assertion: Hydrogen atom consists of anly one electron but its emissio...
Text Solution
|
- (A) bohr model can not be extended to two or more electron atoms. (R...
Text Solution
|
- Assertion: Bohr had to postulate that the electrons in stationary orbi...
Text Solution
|
- (A) bohr's third postulaate states that the stationary orbits are thos...
Text Solution
|
- Assertion: Electrons in the atom are held due to coulomb forces. Rea...
Text Solution
|