A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SOLUTIONS -Assertion And Reason
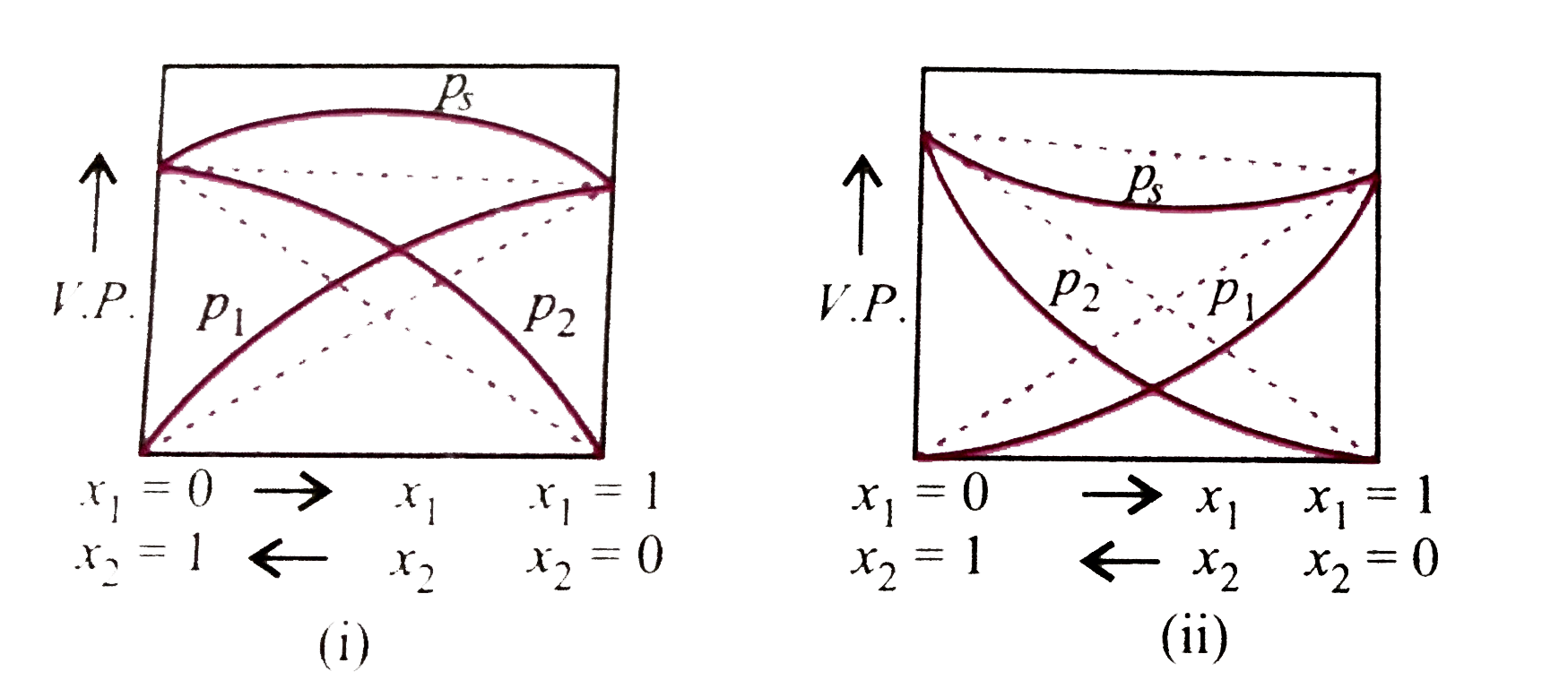
- Study the figures given below and mark the correct statement.
Text Solution
|
- Assertion:Amalgam of mercury with sodium is an example of solid soluti...
Text Solution
|
- Assertion: The concentration of pollutants in water or atmosphere is o...
Text Solution
|
- Assertion: One molar aqueous solution is more concentrated than that o...
Text Solution
|
- Assertion:Pressure does not have any effect on solubility of solids in...
Text Solution
|
- Assertion: Aquatic species are more comfortable in warm waters than co...
Text Solution
|
- Assertion:At equilibrium , vapour phase will be always rich in compone...
Text Solution
|
- Assertion: Decrease in the vapour pressure of water by adding 1 mol of...
Text Solution
|
- Assertion:In an ideal solution , Delta"mix"H is zero Reason :In an ...
Text Solution
|
- Assertion: A solution of phenol and aniline will show negative deviati...
Text Solution
|
- Assertion:The solutions which show large positive deviations from Raou...
Text Solution
|
- Assertion:The vapour pressure of an aqueous solution of sucrose is les...
Text Solution
|
- Assertion:Lowering of vapour pressure is not dependent on the number o...
Text Solution
|
- Assertion:Osmosis does not take place in two isotonic solutions separa...
Text Solution
|
- Assertion: 1 M solution of KCl has greater osmotic pressure than 1 M ...
Text Solution
|
- Assertion:Molecular mass of KCl calculated on the basis of colligative...
Text Solution
|