A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Abnormal Molar Masses|16 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinking Skills|10 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Ideal And Non-Ideal Solutions|13 VideosPRACTICE PAPER -3
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|50 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SOLUTIONS -Colligative Properties And Determination Of Molar Mass
- Study the following figure showing osmosis and mark the correct statem...
Text Solution
|
- Relative lowering of vapour pressure , osmotic pressure of a solution ...
Text Solution
|
- The osmotic pressure of a solution can be increased by
Text Solution
|
- People taking lot of salt experience puffiness or swelling of the body...
Text Solution
|
- The preservation of meat by salting and of fruits by adding sugar prot...
Text Solution
|
- Sea water is desalinated to get fresh water by which of the following ...
Text Solution
|
- A 5% solution of cane sugar (molecular weight=342) is isotonic with 1%...
Text Solution
|
- Which of the following statements is correct about diffusion and osmos...
Text Solution
|
- 10% solution of urea is isotonic with 6% solution of a non-volatile so...
Text Solution
|
- A solution containing 10.2 g of glycrine per litre is found to be isot...
Text Solution
|
- What will be the osmotic pressure in pascals exerted by a solution pre...
Text Solution
|
- Osomotic pressure of a solution containing 2 g dissolved protein per 3...
Text Solution
|
- Twenty grams of a substance were dissolved in 500ml. Of water and the ...
Text Solution
|
- Which of the following statements is not correct ?
Text Solution
|
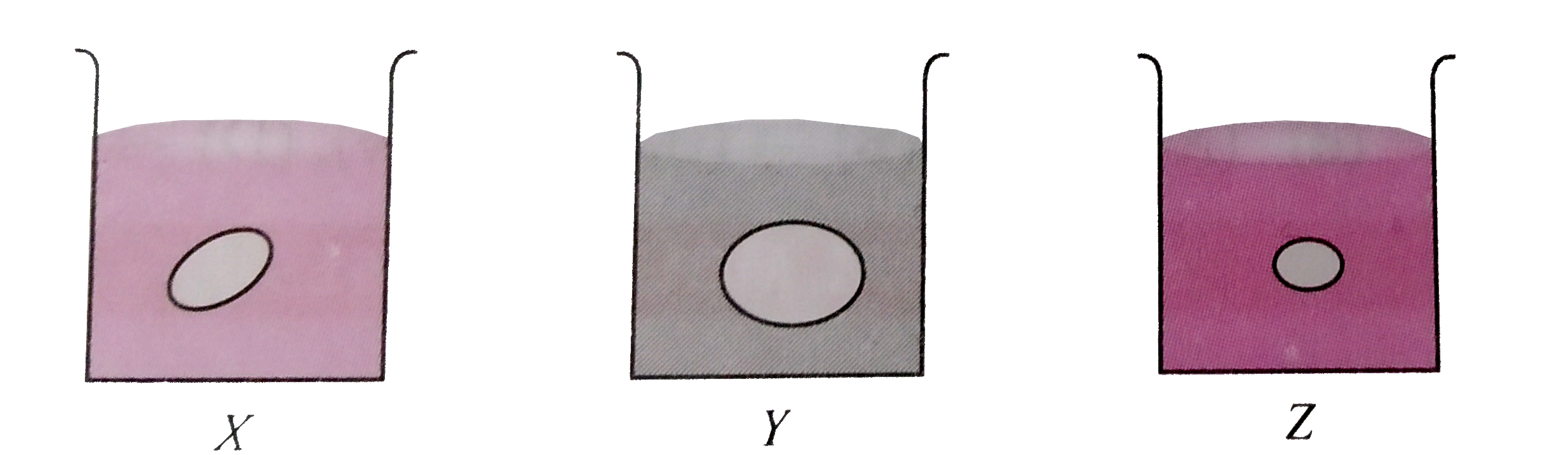
- Grapes placed in three beakers X ,Y and Z containing different type of...
Text Solution
|
- A plant cell shrinks when it is kept in
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- For carrying reverse osmosis for desalination of water the material us...
Text Solution
|
- Which of the following is not an industrial or biological importance o...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|