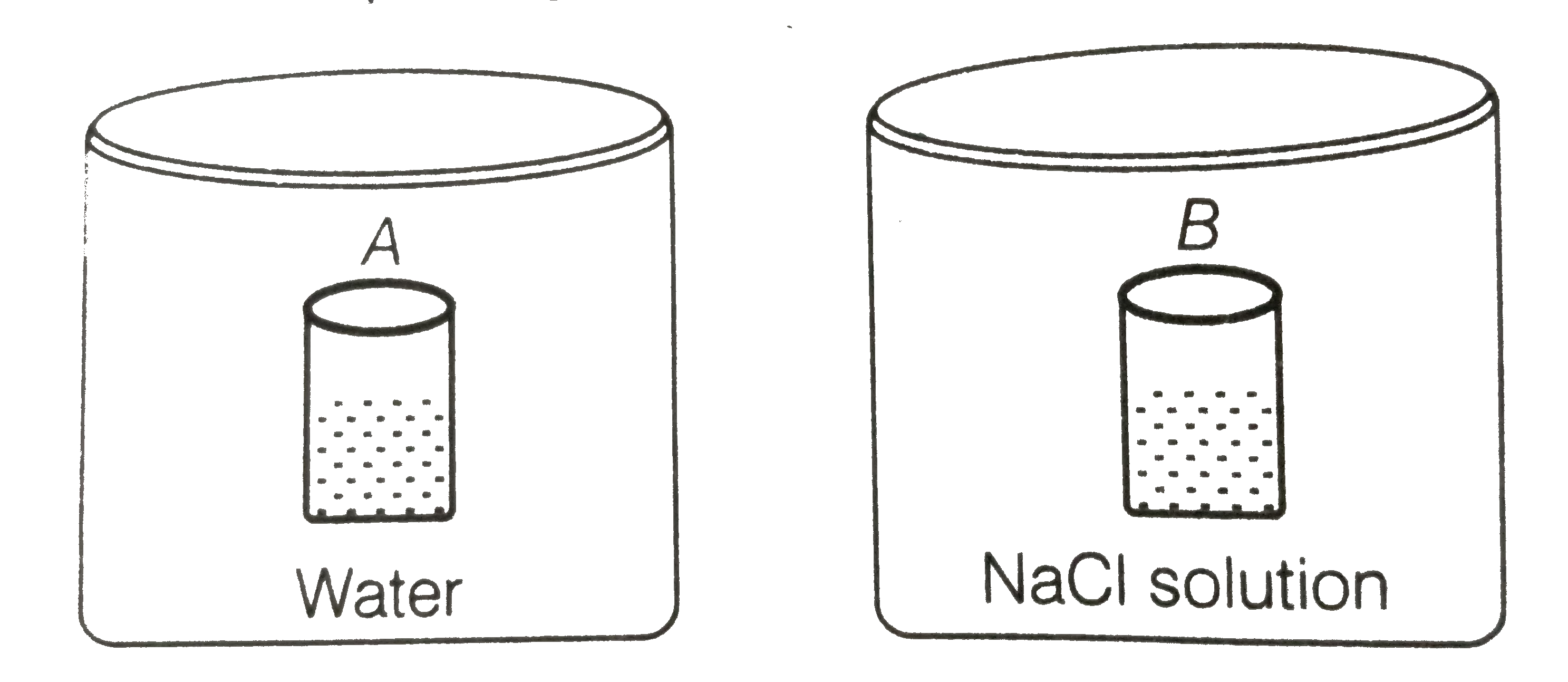
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinking Skills|10 VideosPRACTICE PAPER -3
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|50 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SOLUTIONS -NCERT Exemplar
- Considering the formation, breaking and stregth of hydrogen bond, prad...
Text Solution
|
- Colligative properties depend on
Text Solution
|
- Which of the following aqueous solution should have the highest boilin...
Text Solution
|
- The unit of ebillioscopic constant is
Text Solution
|
- In coparison to a 0.01 M solution of glucose, the depression in freezi...
Text Solution
|
- An unriped mango placed in a concentrated salt solution to prepare pic...
Text Solution
|
- At a given temperature, osmotic pressure of a concentrated solution of...
Text Solution
|
- Which of the following statements is false ?
Text Solution
|
- The values of van't Hoff factors for KCl, NaCl and K2SO4 , respectivel...
Text Solution
|
- Which of the following statements is false ?
Text Solution
|
- Value of Henry's constant K(H)…
Text Solution
|
- Value of Henry's constant K(H)…
Text Solution
|
- Consider the figure and mark the correct option.
Text Solution
|
- We have three aqueous solutions of NaCl labelled as A, B and C with co...
Text Solution
|
- On the basic of information given below mark the Correct option .Infor...
Text Solution
|
- Two beakers of capacity 500 mL were taken. One of these beakers, label...
Text Solution
|
- If two liquids A and B from minimum boiling azeotrope at some specific...
Text Solution
|
- 4 L of 0.02 M aqueous solution of NaCl was diluted by adding 1 L of wa...
Text Solution
|
- On the basis of information given below mark the correct option. Inf...
Text Solution
|
- K(H) value for Ar(g),CO(g), HCHO(g) and CH(4)(g) are 40.39 , 1.67 ,1....
Text Solution
|