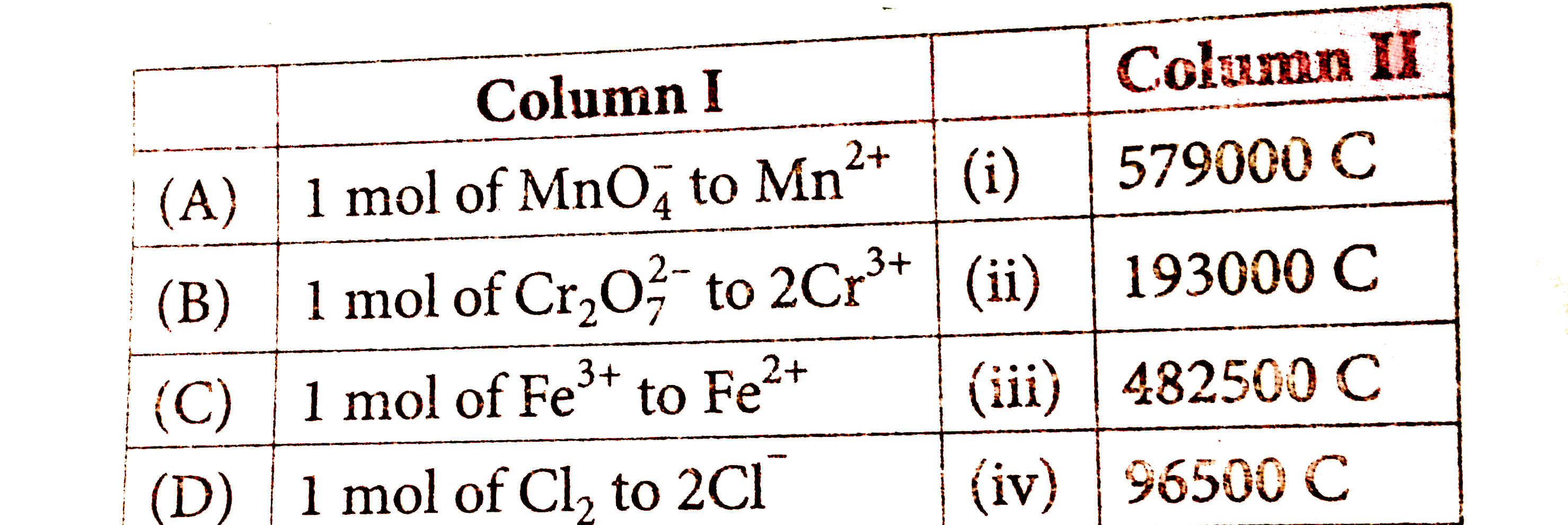A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Batteries|2 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Fuel Cells|1 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Conductance Of Electrolytic Solutions|23 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ELECTROCHEMISTRY-Electrolytic Cells And Electrolysis
- If a current of 1.5 ampere flows through a metallic wire for 3 hours, ...
Text Solution
|
- How many coulombs of electricity is required to reduce 1 mole of Cr2O7...
Text Solution
|
- An electric charge of 5 Faradays is passed through three electrolytes...
Text Solution
|
- A current of 1.40 ampere is passed through 500 mL of 0.180 M solution ...
Text Solution
|
- How much time is required to deposit 1xx10^(-3) cm thick layer of sil...
Text Solution
|
- How much metal will be deposited when a current of 12 ampere with 75% ...
Text Solution
|
- Same amount of electric current is passed through the solutions of AgN...
Text Solution
|
- When during electrolusis of a solution of AgNO3, 9650 coulmbs of char...
Text Solution
|
- The amount of chlorine evolved by passing 2 A of current in an aqueous...
Text Solution
|
- How many moles of Pt may be deposited on the cathode when 0.80F of ele...
Text Solution
|
- An electric current is passed through silver nitrate solution using si...
Text Solution
|
- If 54 g of silver is deposited during an electrolysis reaction, how mu...
Text Solution
|
- Standard electrode potentials of few half-cell reactions are given bel...
Text Solution
|
- An acidic solution of Cu^(2+) ions containing 0.4 g of Cu^(2+) ions is...
Text Solution
|
- Choose the option with correct words to fill in the blanks. Accordi...
Text Solution
|
- Which of the following statement is true?
Text Solution
|
- During the electrolysis of dilute sulphuric acid, the following proces...
Text Solution
|
- In electrolysis of dilute H(2)SO(4) what is liberated at anode?
Text Solution
|
- When an aqueous solution of AgNO3 is electroysed between platinum elec...
Text Solution
|
- Electrolysis of an aqueous solution of AgNO3 with silver electrodes pr...
Text Solution
|