A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise HOTS|10 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise EXEMPLAR PROBLEMS|17 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ELECTROCHEMISTRY-Assertion And Reason
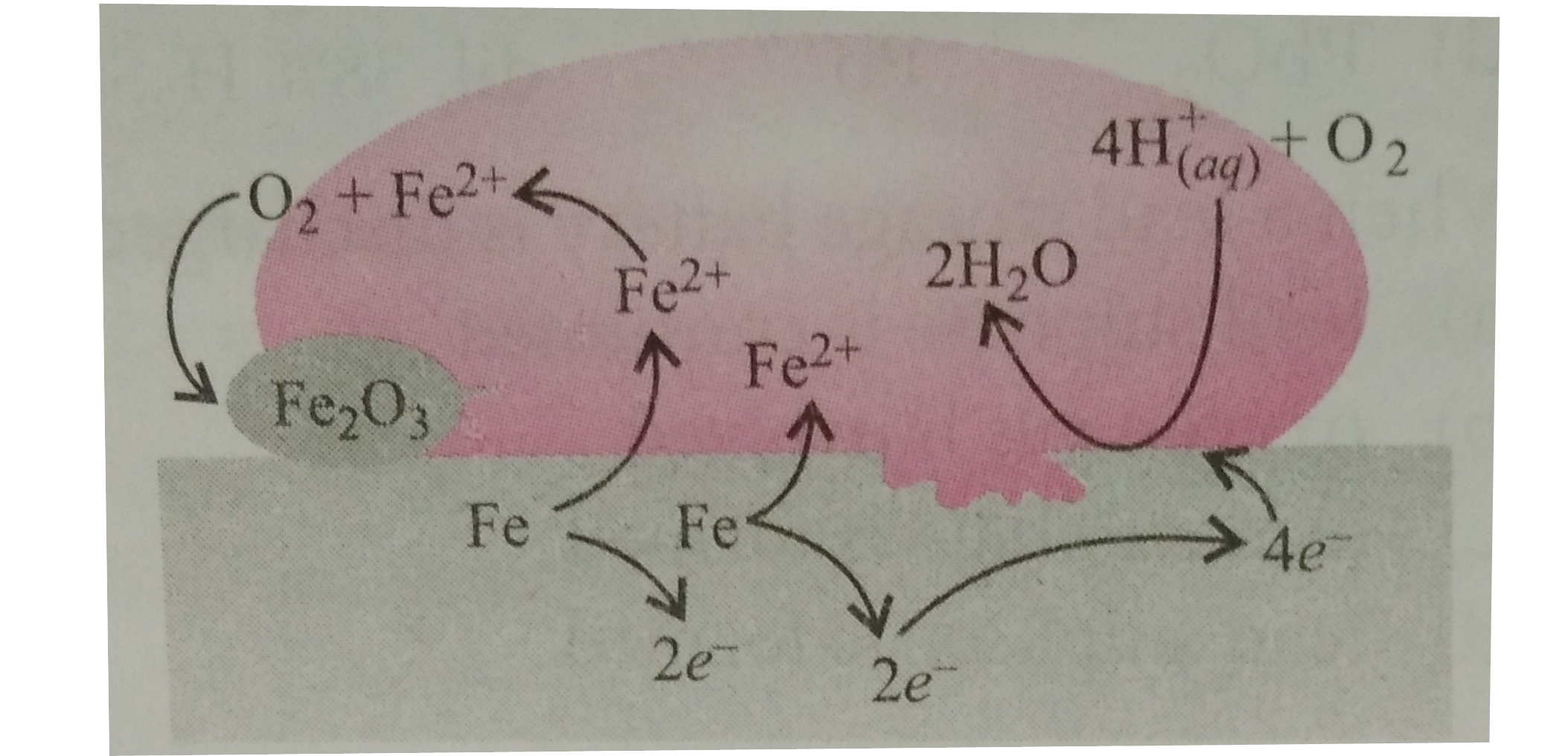
- The given figure shows the corrosion of iron in atmosphere. Fill ...
Text Solution
|
- Assertion:Electrolytic cell uses electrical energy to carry non-sponta...
Text Solution
|
- Assertion :EMF of the cell is the potential difference between the ele...
Text Solution
|
- Assertion:A standard hydrogen electrode is also called reversible elec...
Text Solution
|
- Assertion:Cu^(2+) ions get reduced more easily than H^+ ions. Reaso...
Text Solution
|
- Assertion:Lithium has the lowest electrode potential. Reason: Lithiu...
Text Solution
|
- Assertion : Current stops flowing when E(cell)=0. Reason : ...
Text Solution
|
- Assertion : To obtain maximum work from a galvanic cell charge has to...
Text Solution
|
- Assertion:The electrical resistance of any object decrease with increa...
Text Solution
|
- Assertion: The conductivity of electrolytic soutions increase with inc...
Text Solution
|
- Assertion:Molar conductivity increases with decrease in concentration....
Text Solution
|
- Assertion:Kohlrausch law helps to find the molar conductivity of weak ...
Text Solution
|
- Assertion:When a copper wire is dipped in silver nitrate solution, the...
Text Solution
|
- Assertion : In electrolysis, the quantity of electricity needed for de...
Text Solution
|
- Assertion:In electrolysis of aqueous NaCl the product obtained is H2 g...
Text Solution
|
- Assertion:In mercury cell, the cell potential is approximately 1.35 V ...
Text Solution
|