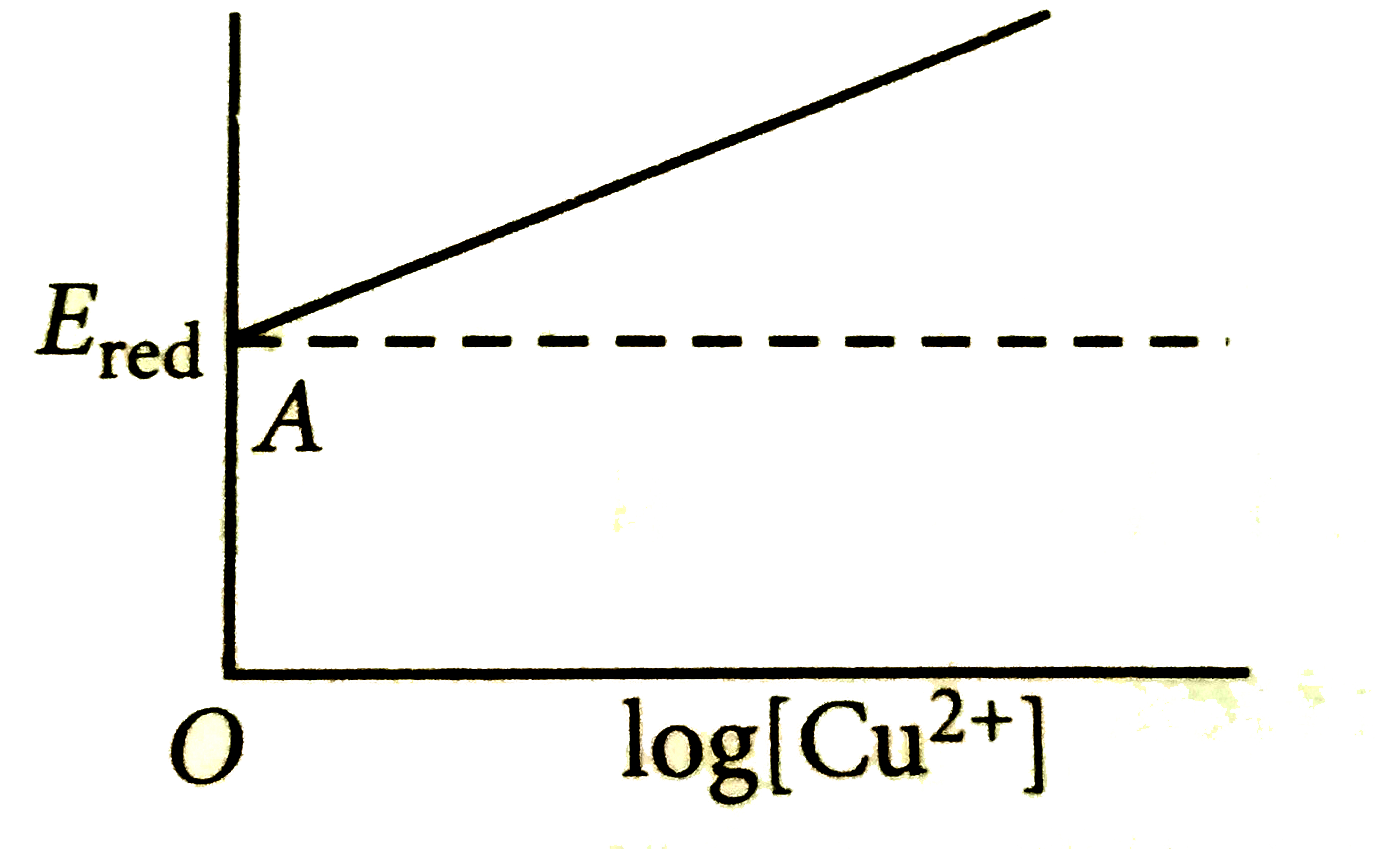A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise EXEMPLAR PROBLEMS|17 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ELECTROCHEMISTRY-HOTS
- Which of the following reactions canot be a base for electrochemical c...
Text Solution
|
- In a galvanic cell, the salt bridge (i) does not participate chemica...
Text Solution
|
- The position of some metals in the electrochemical series in dectreasi...
Text Solution
|
- A gas X at 1 atm is bubbled through a solution containing a mixture of...
Text Solution
|
- For the cell prepared from electrodes A and B, Electrode A :Cr2O7^(...
Text Solution
|
- The formal potential of Fe^(3+)//Fe^(2+) in a sulphuric acid and phosp...
Text Solution
|
- Calculate the euilibrium constant for the reaction, 2Fe^(3+) + 3I^(-...
Text Solution
|
- The emf of a cell corresponding to the reaction Zn +2H^(+)(aq) rarr ...
Text Solution
|
- For the reaction, Cu^(2+)+2e^(-) to Cu , log [Cu^(2+)] vs E graph is ...
Text Solution
|
- How long will it take for a uniform current of 6.0 ampere to deposit 7...
Text Solution
|