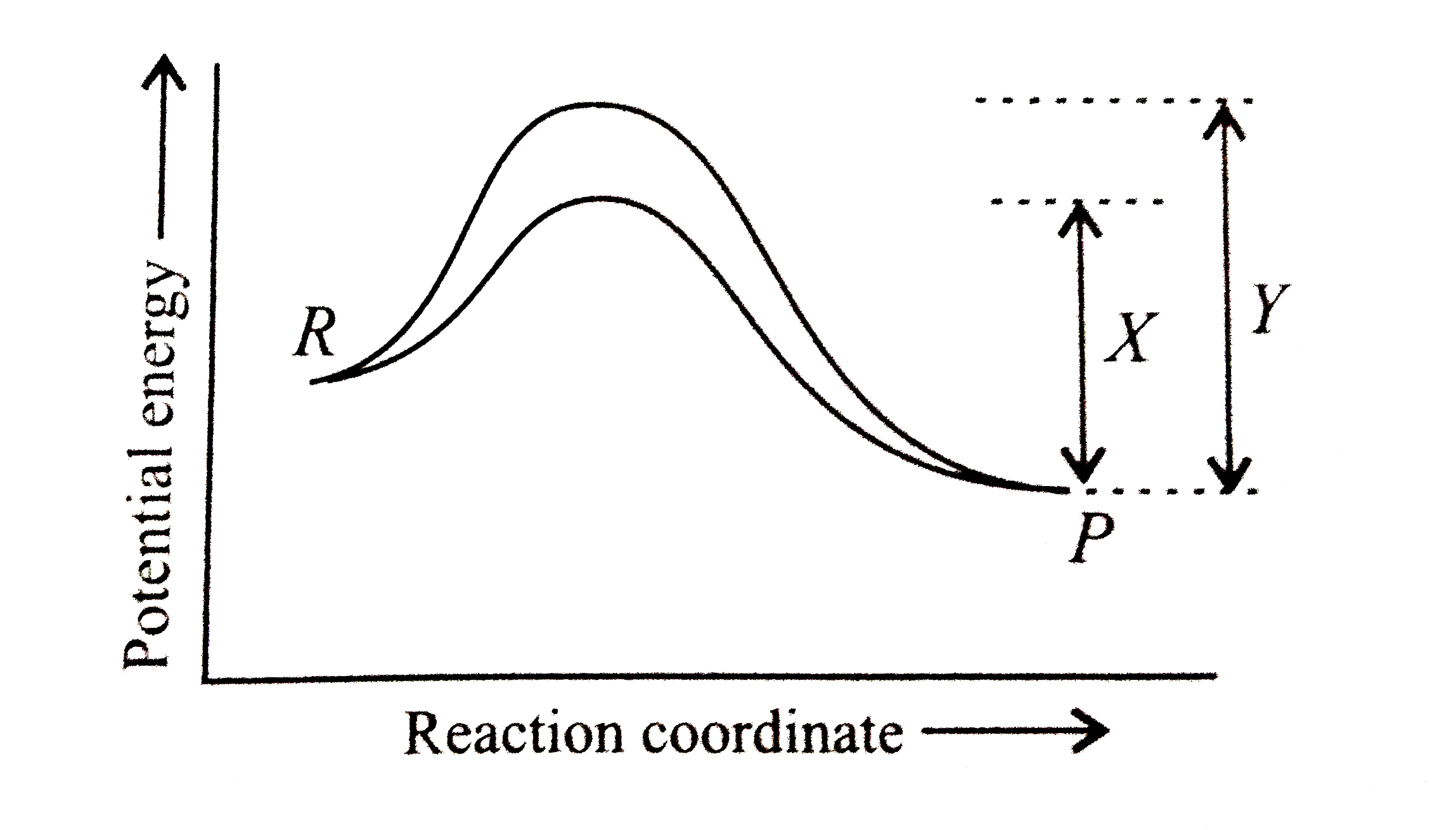
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise Collision Theory Of Chemical Reactions|6 VideosCHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|20 VideosCHEMICAL KINETICS
NCERT FINGERTIPS ENGLISH|Exercise Integrated Rate Equation|25 VideosBIOMOLECULES
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-CHEMICAL KINETICS-Temperature Dependendence Of The Rate Of A Reaction
- The Activation energy for a chemical reaction mainly depends upon
Text Solution
|
- The temperature dependence of the rate of a chemical reaction can be e...
Text Solution
|
- The energy diagram of a reaction P + Q to R + S is given. What are A...
Text Solution
|
- The potential energy diagram for a reaction X to Y is given. A and C i...
Text Solution
|
- An endothermic reaction with high activation energy for the forward re...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- If hydrogen and oxygen are mixed and kept in the same vessel at room t...
Text Solution
|
- The temperature dependence of the rate of a chemical reaction is given...
Text Solution
|
- The temperature dependence of the rate constant k is expressed as k = ...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- The decomposition of hydrocarbon follows the equation k=(4.5xx10^(11)...
Text Solution
|
- The rate constant for a first order reaction at 300^(@)C for which E(a...
Text Solution
|
- The graph of the effect of catalyst on activation energy is given belo...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- Which of the following statement about the catalyst is/are true?
Text Solution
|
- When a catalyst is used an equilibrium process,
Text Solution
|
- Which of the following factors are responsible for the increase in the...
Text Solution
|