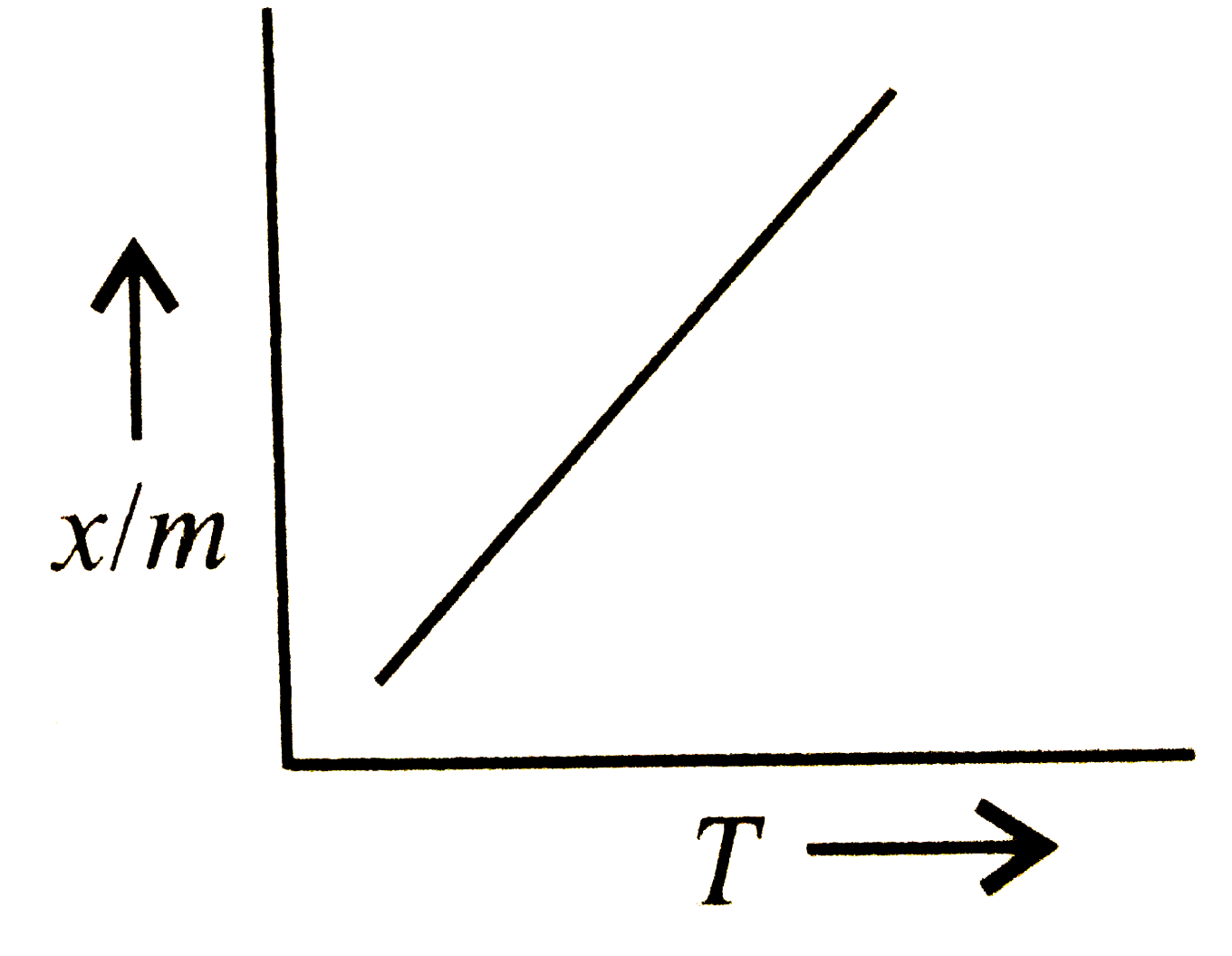
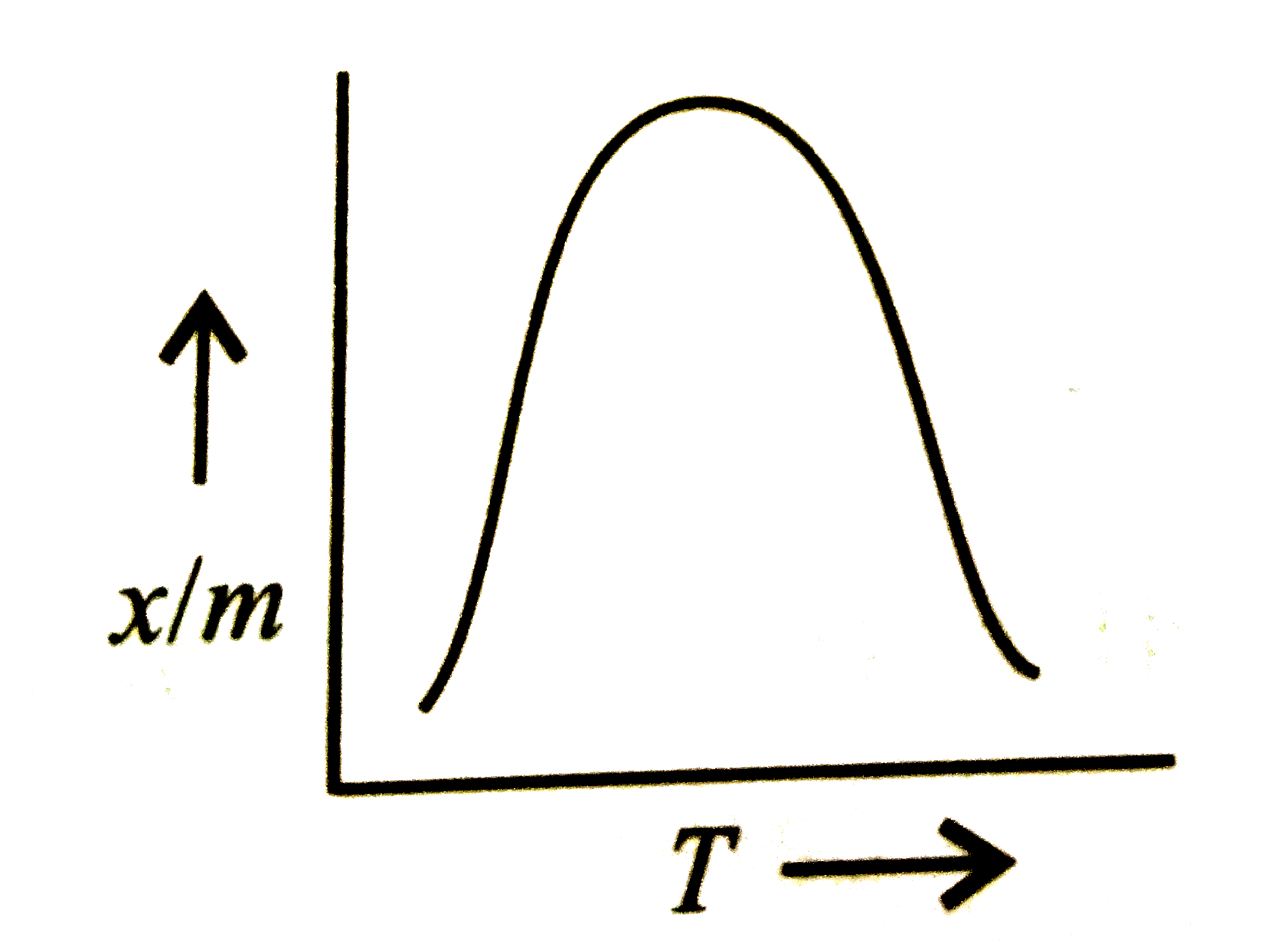
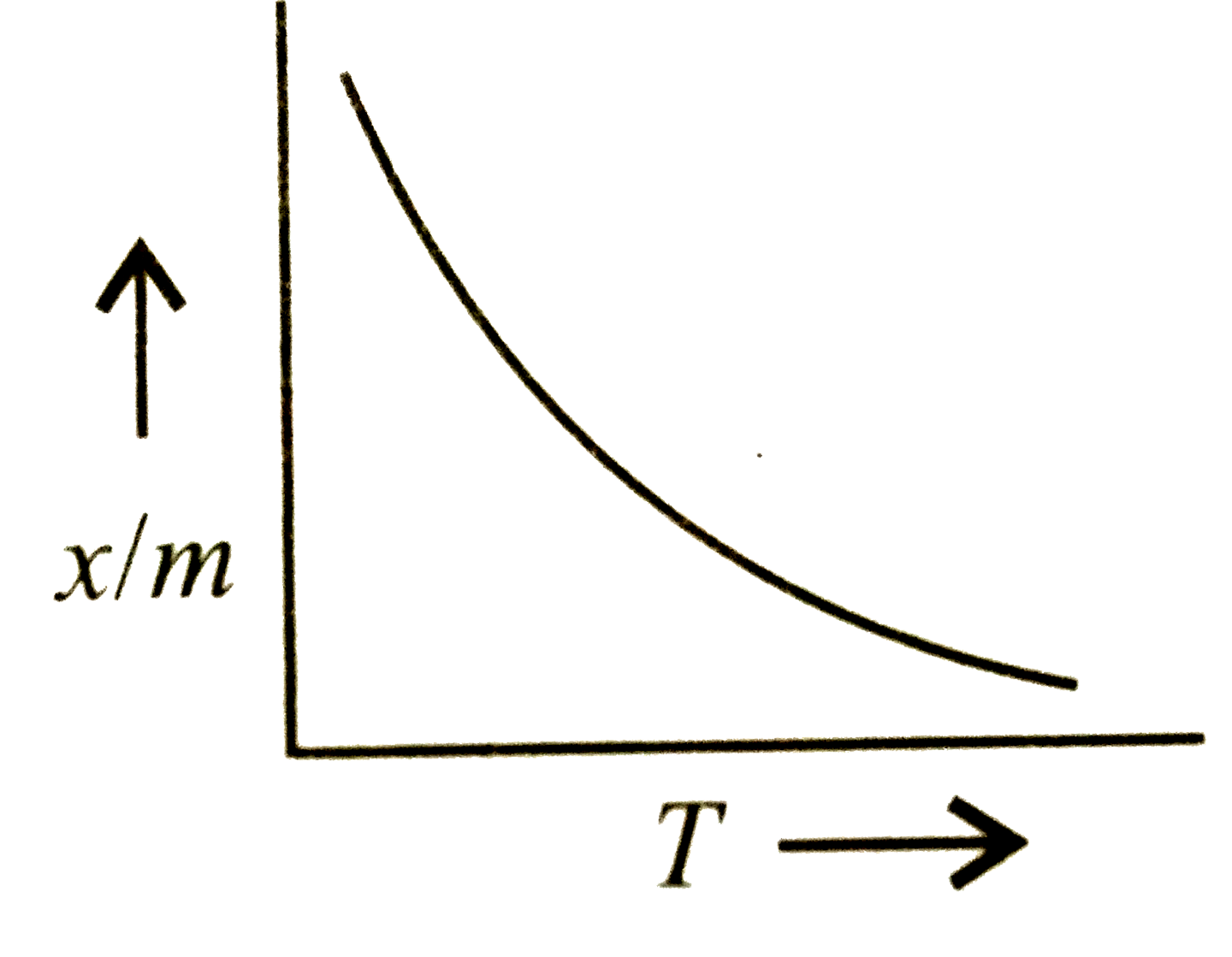
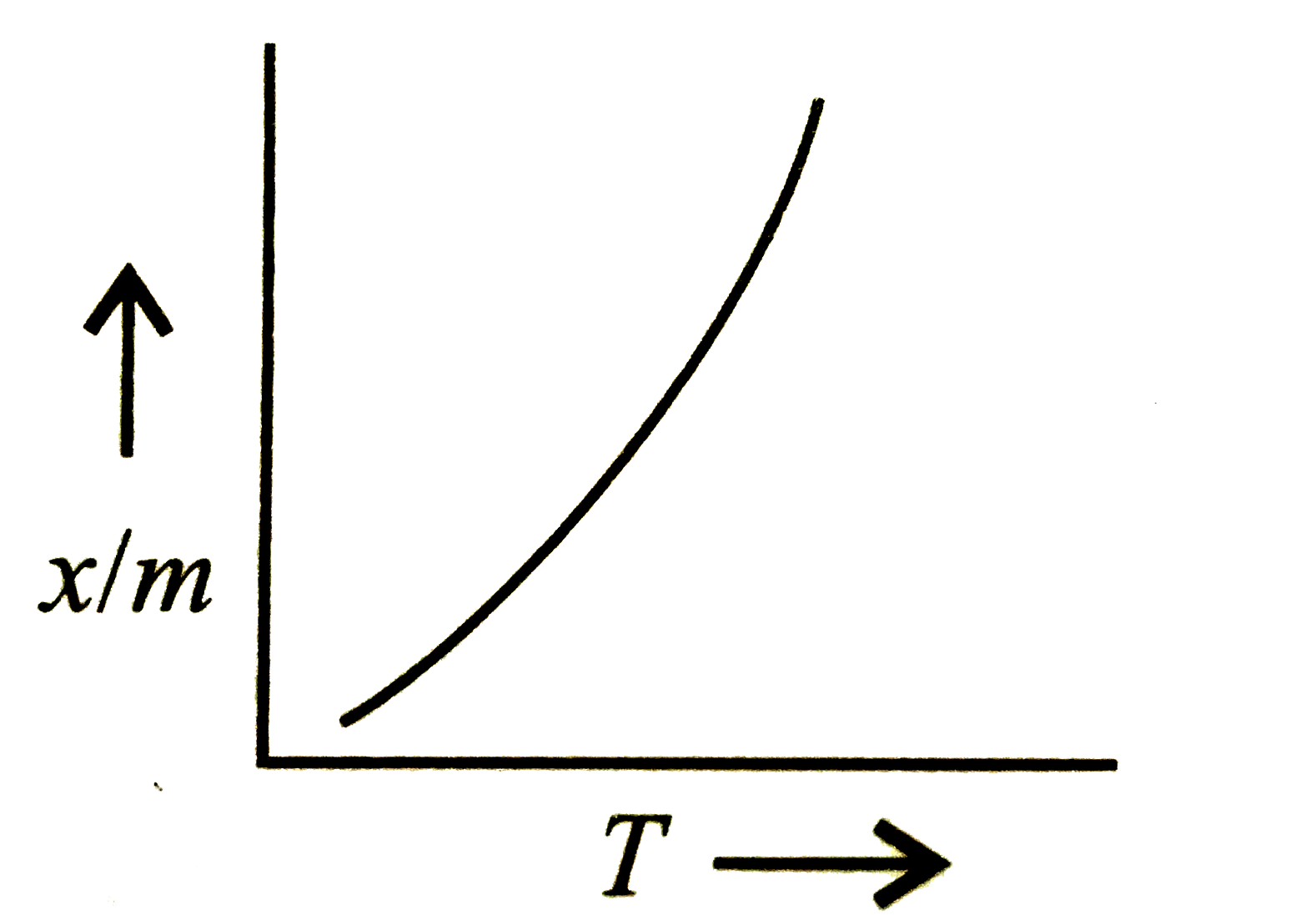
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise NCERT EXEMPLAR PROBLEMS|25 VideosSOLUTIONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-SURFACE CHEMISTRY-Assertion And Reason
- Which plot is the adsorption isobar for chemisorption?
Text Solution
|
- Assertion : Solids in finely divided state act as good adsorbents. ...
Text Solution
|
- Assertion : Silica gel is used to dry air. Reason : Silica gel absor...
Text Solution
|
- Assertion : In physical adsorption , enthalpy of adsorption in very lo...
Text Solution
|
- Assertion : Physical adsorption increases with increase in temperature...
Text Solution
|
- Assertion : Physisorption of a gas adsorbed at low temperature may cha...
Text Solution
|
- Assertion : Hydrolysis of ester is an example of auto-catalytic reacti...
Text Solution
|
- Assertion : Zeolites are good shape-selective catalyst. Reason : Ze...
Text Solution
|
- Assertion : Amylase in the presence of sodium choride i.e., Na^(+) ion...
Text Solution
|
- Assertion : Lyophilic sols are reversible sols. Reason : Lyophilic...
Text Solution
|
- Assertion : Colloidal sol scatters light while true solution does not....
Text Solution
|
- Assertion : The values of colligative properties are of smaller order ...
Text Solution
|
- Assertion : When KI solution is added to AgNO(3) solution, negatively ...
Text Solution
|
- Assertion : In the coagulation of a negative sol the flocculating powe...
Text Solution
|
- Assertion : Lyophilic colloids have a unique property of protecting ly...
Text Solution
|
- Assertion : For stabilisation of an emulsion a third component called ...
Text Solution
|