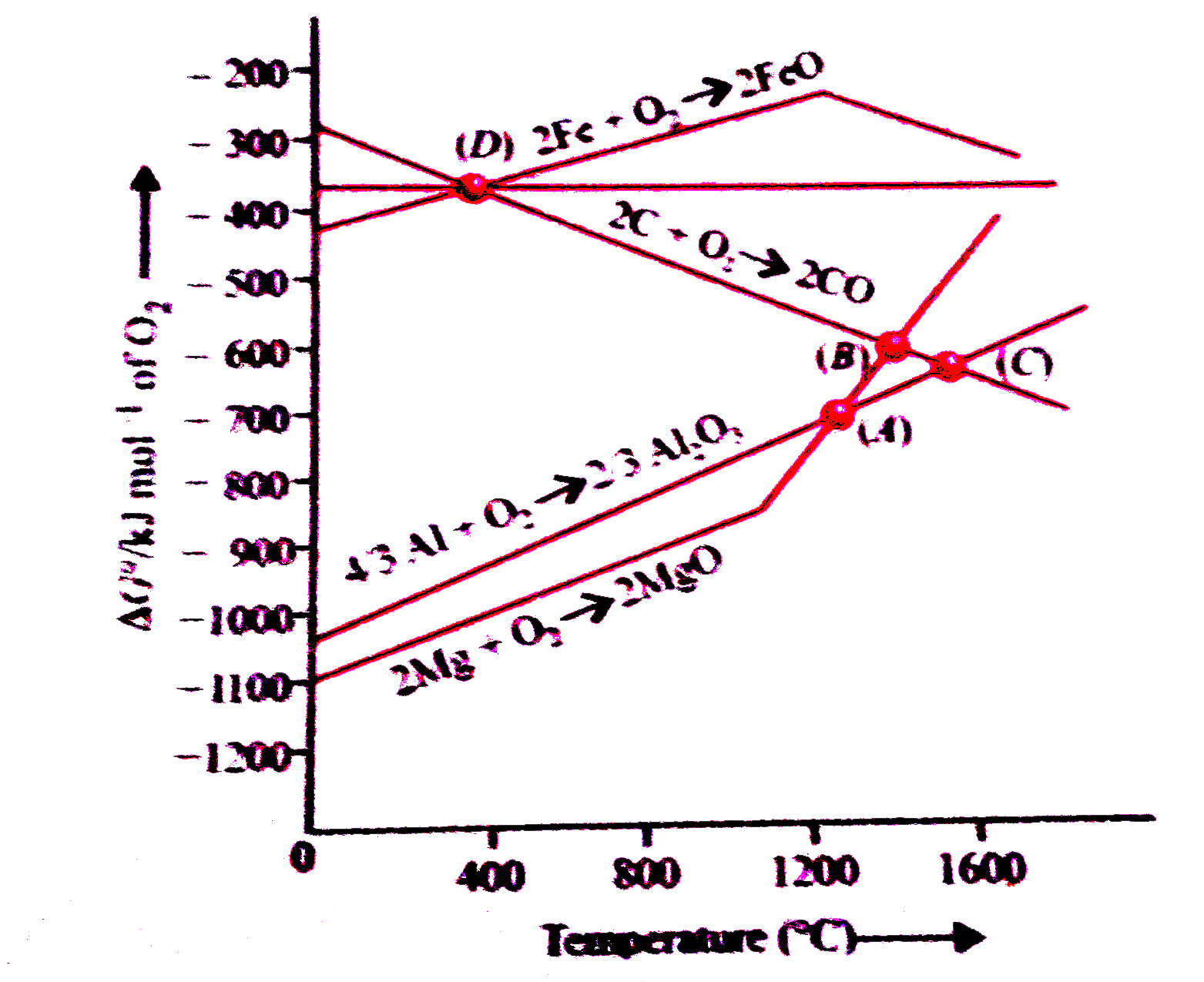
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise NCERT EXEMPLAR PROBLEMS|13 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosHALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|27 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS-Assertion And Reason
- At the points of intersection of Al(2)O(3) and MgO curves (A), DeltaG^...
Text Solution
|
- Assertion : Minerals are naturally occurring chemical substances in t...
Text Solution
|
- Assertion : In froth floatation method, collectors such as pine oil or...
Text Solution
|
- Assertion : Gold and silver are extracted from their native ores by le...
Text Solution
|
- Assertion: Roasting is a process in which the ore is heated in presenc...
Text Solution
|
- Assertion : Reduction of a metal oxide is easier if the metal formed i...
Text Solution
|
- Assertion : Sulphide ores are converted to oxides before reduction. R...
Text Solution
|
- Assertion : Magnesium metal is not used for the reduction of alumina i...
Text Solution
|
- Assertion : In the metallury of aluminium , purified Al(2)O(3) is mixe...
Text Solution
|
- Assertion: Limestone addes in the blast furnace decomposes to give CaO...
Text Solution
|
- Assertion : Tin is refined by liquation method. Reason : Tin has low...
Text Solution
|
- Assertion : In electrolytic refining of metal, impure metal is made ca...
Text Solution
|
- Assertion: Zone refining method is used to produce pure metals which a...
Text Solution
|
- Assertion: Nickel is purified by reaction it with CO. Reason: Impur...
Text Solution
|
- Assertion : van Arkel method is used for refining of Zinc. Reason :...
Text Solution
|
- Assertion : Chromatography in general involves a mobile (a gas, a liqu...
Text Solution
|