A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Occurrence Of Metals|13 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise HOTS|7 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosHALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|27 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS-NCERT EXEMPLAR PROBLEMS
- In the extraction of chlorine by electrolysis of brine.
Text Solution
|
- When copper ore is mixed with silica in a reverberatory furnace, coppe...
Text Solution
|
- Which of the following reaction is an example of autoreduction ?
Text Solution
|
- A number of elements are available in earth's crust but most abundant ...
Text Solution
|
- Zone refining is based on the principle that
Text Solution
|
- In the extraction of Cu from its sulphide ore, the metal is formed by ...
Text Solution
|
- Brine is electrolysed by using inert electrodes. The reaction at anode...
Text Solution
|
- In the metallurgy of aluminium,
Text Solution
|
- Electrolytic refining is used to purify which of the following metals?
Text Solution
|
- Extraction of gold and silver involves leaching the metal with CN^(-) ...
Text Solution
|
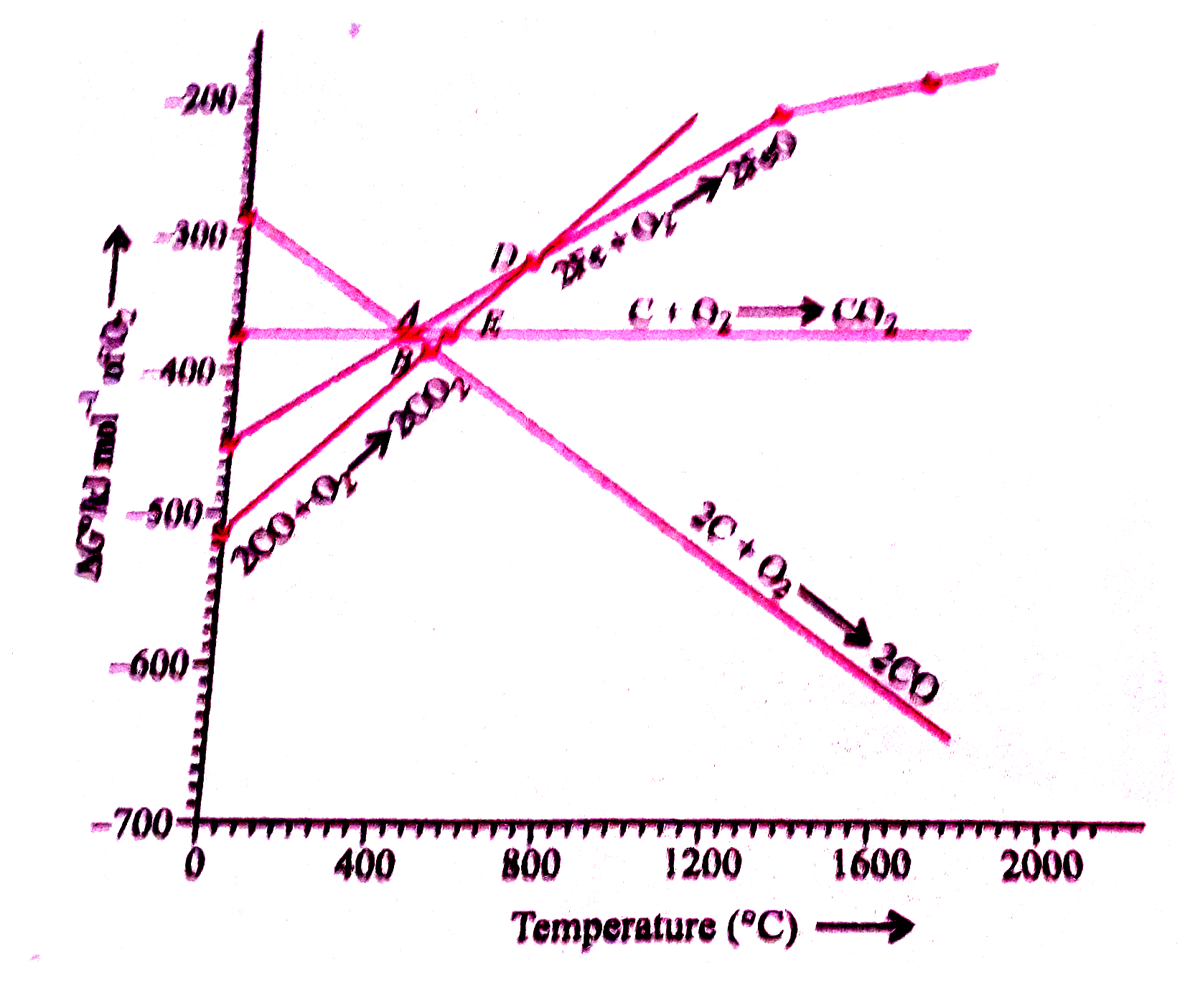
- Choose the correct option of temperature at which carbon reduces FeO t...
Text Solution
|
- Below point 'A' FeO can .
Text Solution
|
- For the reducetion of FeO at the temperature corresponding to point D,...
Text Solution
|