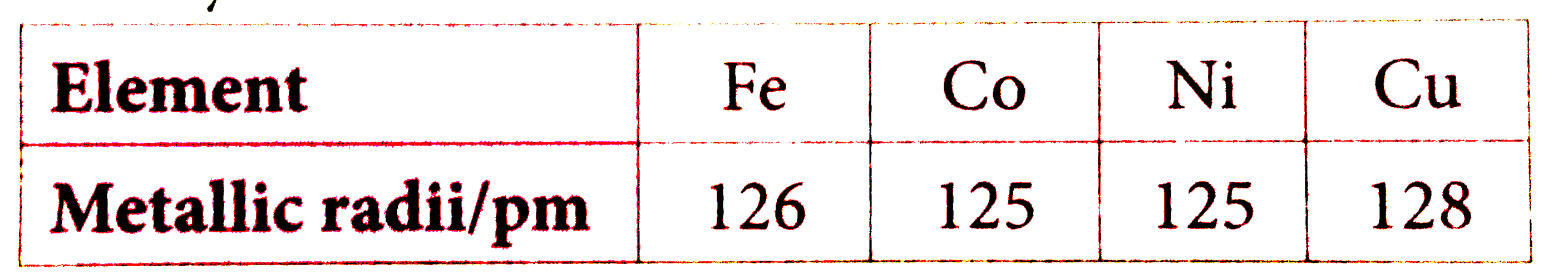A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE D- AND F- BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinking Skills|10 VideosSURFACE CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE D- AND F- BLOCK ELEMENTS -NCERT Exemplar
- Electronic confriguration of a transition element X in +3 oxidation st...
Text Solution
|
- The electronic configurationo of Cu(II) is 3d^(9) whereas that of Cu(I...
Text Solution
|
- Metallic radii of some transition elements are given below. Which of t...
Text Solution
|
- Generally transition elements form coloured salts due to the presence ...
Text Solution
|
- On addition of small amount of KMnO(4) to concentrated H(2)SO(4), a gr...
Text Solution
|
- The magnetic nature of elements depends on the presence of unpaired el...
Text Solution
|
- Which of the following oxidation state si common for all lanthanoids?
Text Solution
|
- Which of the following reactions are disproportionation reactions? ...
Text Solution
|
- When KMnO(4) solution is added to oxalic acid solution , the decolouri...
Text Solution
|
- There are 14 elements in actinoid series. Which of the following eleme...
Text Solution
|
- KMnO(4) acts as an oxidising agent in acidic medium. The number of mol...
Text Solution
|
- Which of the following is an amphoteric oxide?
Text Solution
|
- Gadolinium belongsd to 4f series. It's atomic number is 64. which of t...
Text Solution
|
- Interstitial compounds are formed when small atoms are trapped inside ...
Text Solution
|
- The magnetic moment is associated with its spin angular momentum and o...
Text Solution
|
- KMnO(4) acts as on oxidising agent in alkaline medium. When alkaline K...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- When acidified K(2)Cr(2)O(7) solution is added to Sn^(2+) salts then S...
Text Solution
|
- Higher oxidation state of manganese in fluoride is +4 (MnF(4)) but hig...
Text Solution
|
- Although zirconium belongs to 4d transition series and hafnium to 5d t...
Text Solution
|