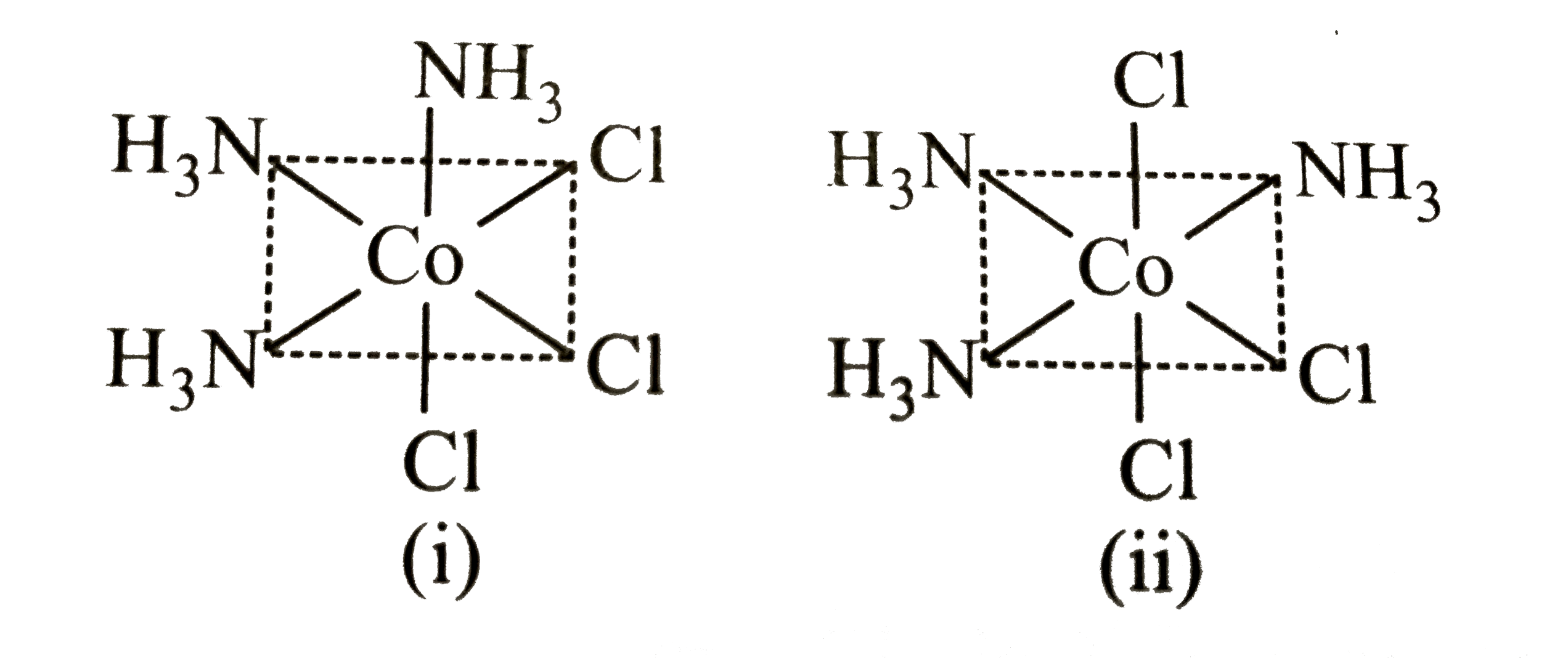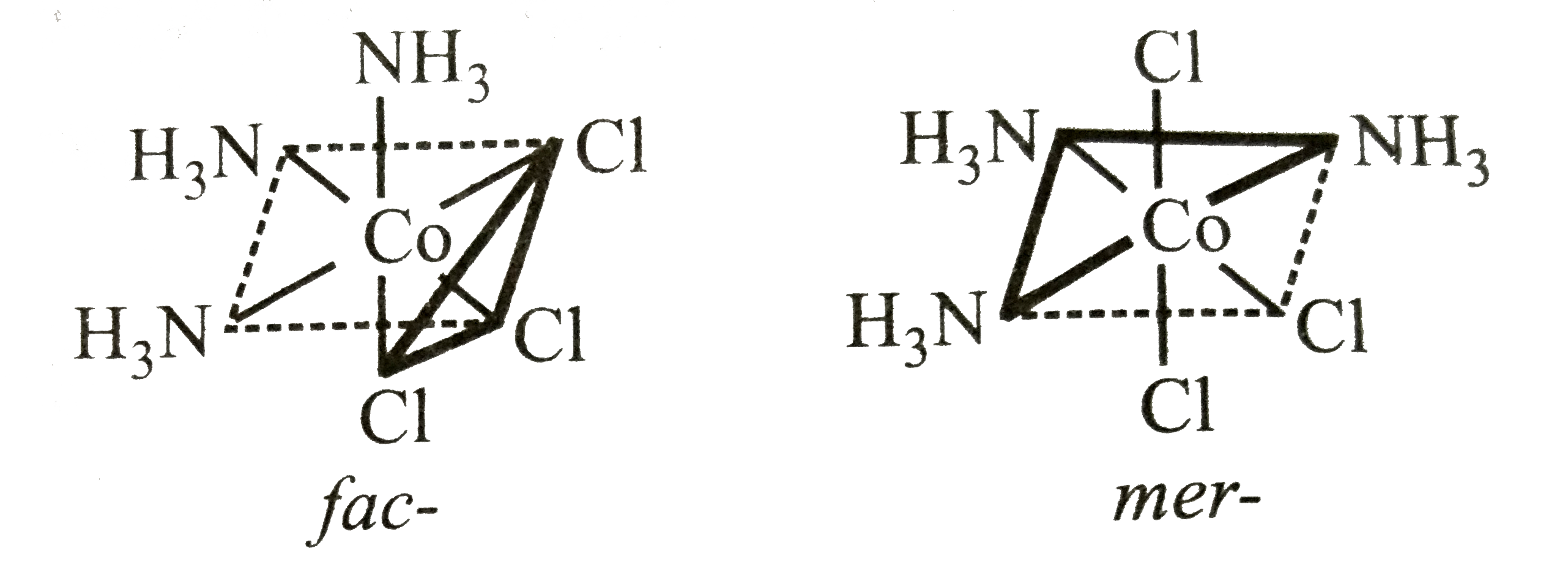A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Bonding In Coordination Compounds|40 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Bonding In Metal Carbonyls|5 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Nomenclature Of Coordination Compounds|9 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|15 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-COORDINATION COMPOUNDS -Isomerism In Coordination Compounds
- How many geometrical isomers are there for (a)[Co(NH(3))(2)CI(4)]^(Θ...
Text Solution
|
- Which of the following will not show geometrical isomerism ?
Text Solution
|
- For the square planar complex [M(a) (b) (c ) (d)] (where M = central m...
Text Solution
|
- Which of the following complex species is not expected to exhibit opti...
Text Solution
|
- [Co(NH(3))Cl(en)(2)]^(2+) exist which isomerism ?
Text Solution
|
- Which of the following gives the maxium number of isomers?
Text Solution
|
- Which of the following pair of compounds is a pair of enantiomers
Text Solution
|
- Two isomers of a compound Co(NH(3))(3)Cl(3) (MA(3)B(3) type ) are show...
Text Solution
|
- Which of the following compounds exhibits linkage isomerism?
Text Solution
|
- [Pt(NH(3))(4)][CuCl(4)] and [Cu(NH(3))(4)][PtCl(4)] are known as
Text Solution
|
- Which of the following complex will give white ppt. with BaCl(2) solut...
Text Solution
|
- The Compounds [Cr(H(2)O)(6)]CI(3) , [Cr(H(2)O)(5)CI]CI(2) . H(2)O and ...
Text Solution
|
- Which of the following pairs of isomers is not correctly matched with ...
Text Solution
|
- CrCl(3) * 6H(2)O exists in different isomeric forms which show differe...
Text Solution
|
- Match the column I and with column II and mark the appropriate choice ...
Text Solution
|
- What type of isomerism exists in the following pairs of complexes ? ...
Text Solution
|