A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|14 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS ENGLISH|Exercise Application Of Coordination Compounds|2 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|15 VideosELECTROCHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-COORDINATION COMPOUNDS -Higher Order Thinking Skills
- If excess of AgNO(3) solution is added to 100mL of a 0.024 M solution ...
Text Solution
|
- 0.02 mole of [Co(NH(3))(5)Br]Cl(2) and 0.02 mole of [Co(NH(3))(5) Cl] ...
Text Solution
|
- Both geometrical and optical isomerism are shown by
Text Solution
|
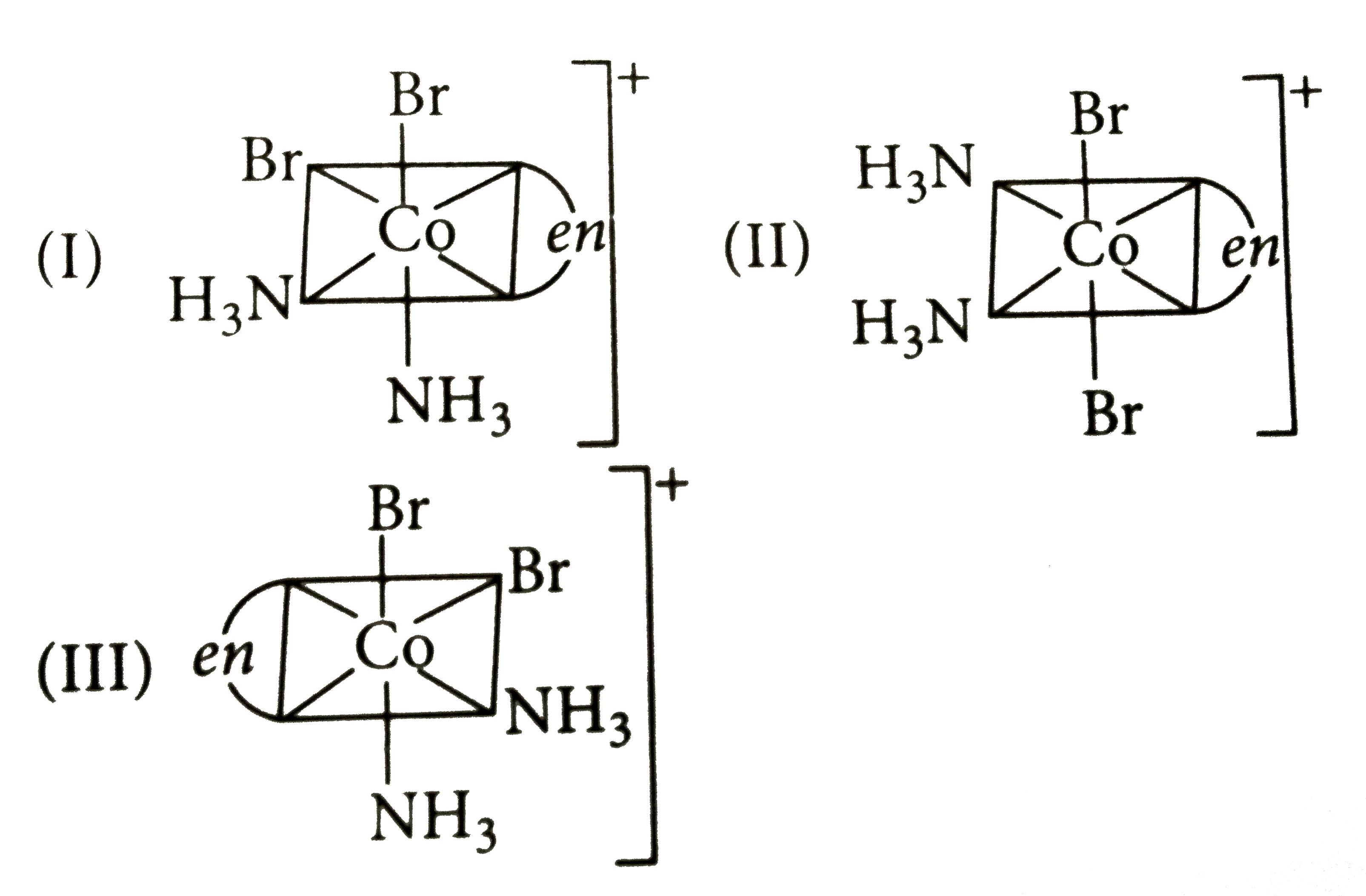
- Three arrangement are shown for the complex , [Co(en) (NH(3))(2) Br(2)...
Text Solution
|
- Among the following complexes (K - P) : K(3) [Fe(CN)(6)] - K , [Co(NH(...
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- Which one of the following has largest number of isomers?
Text Solution
|
- Match each coordination compound in List-I with an appropriate pair of...
Text Solution
|
- Which of the following energy diagrams shows the electron distribution...
Text Solution
|