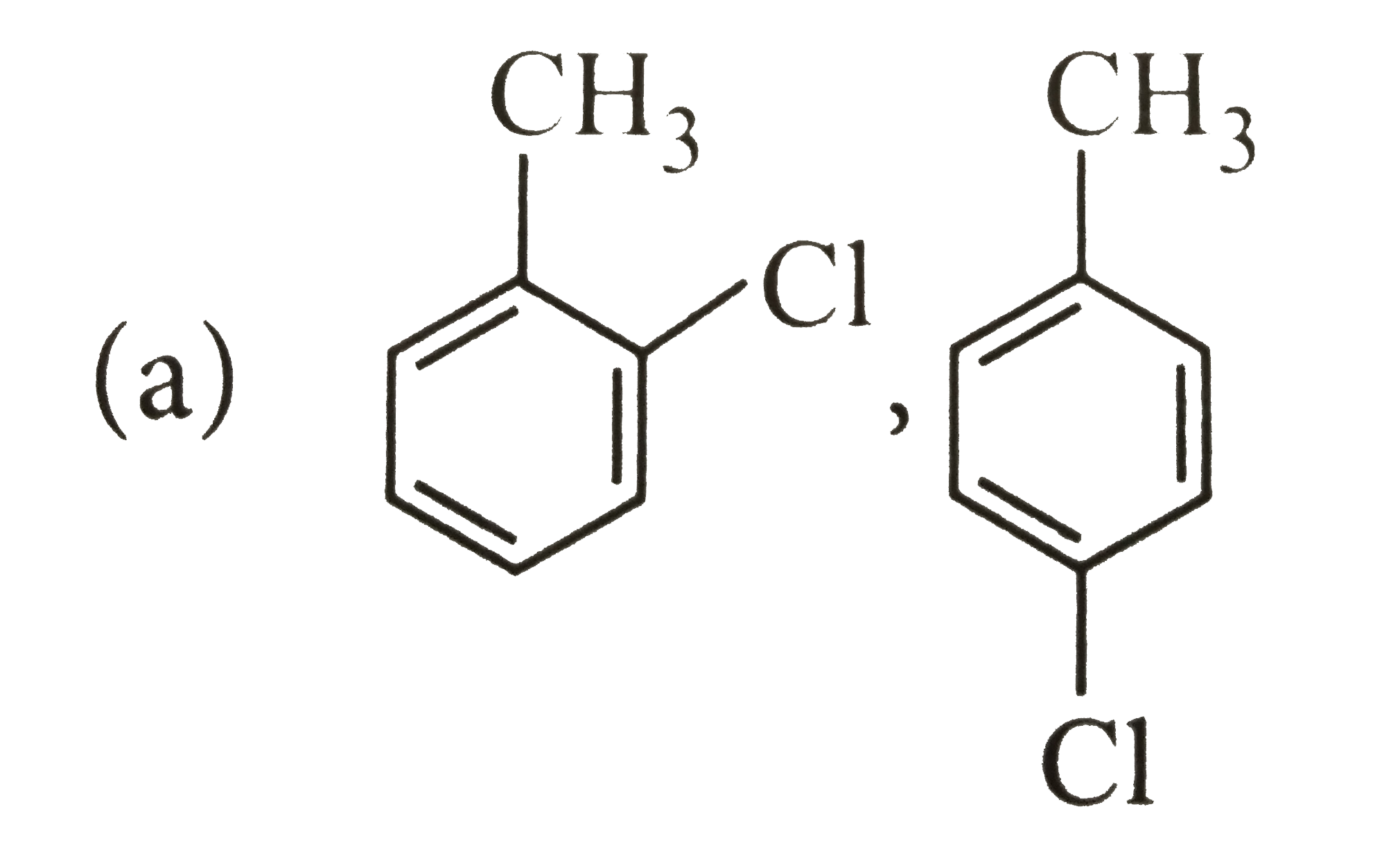
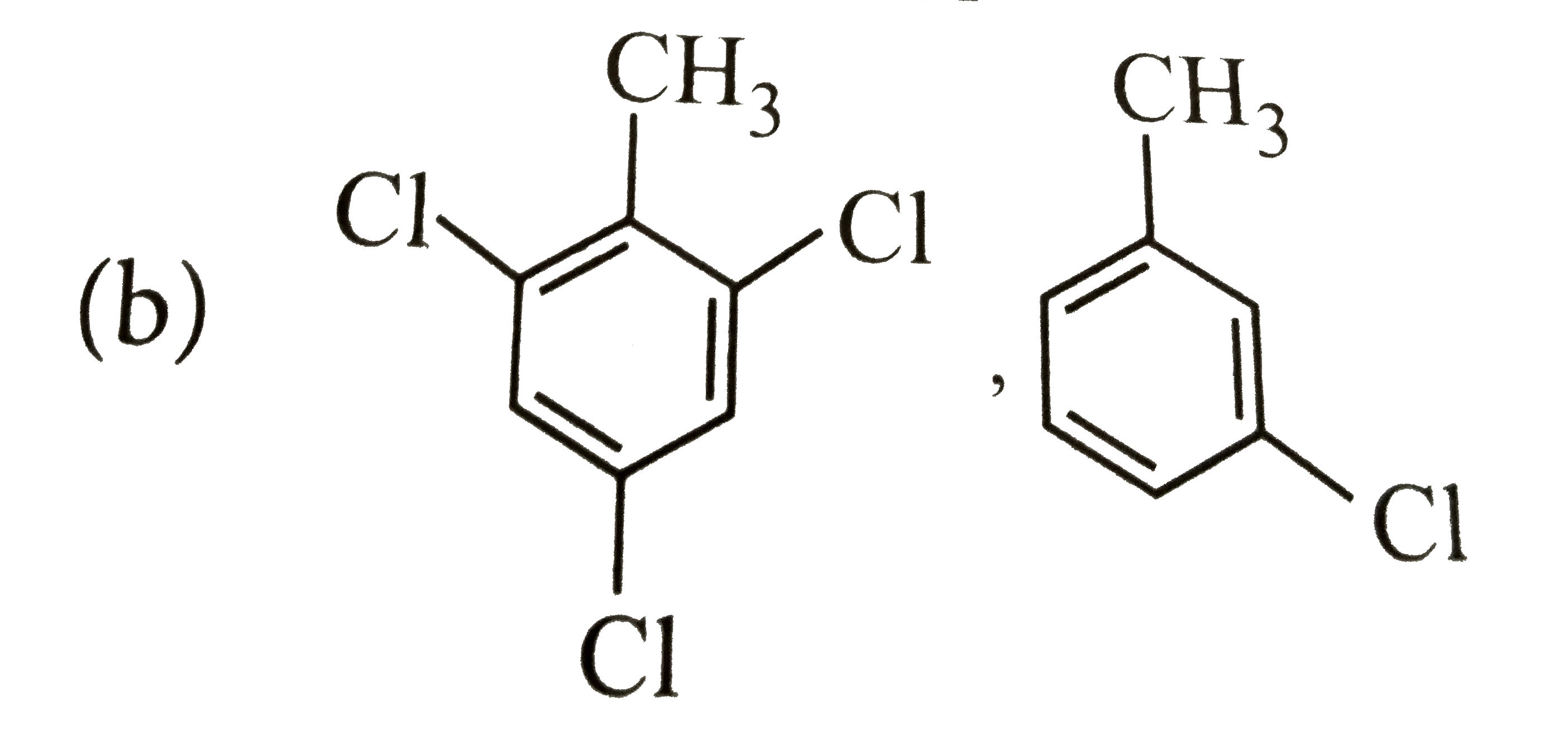
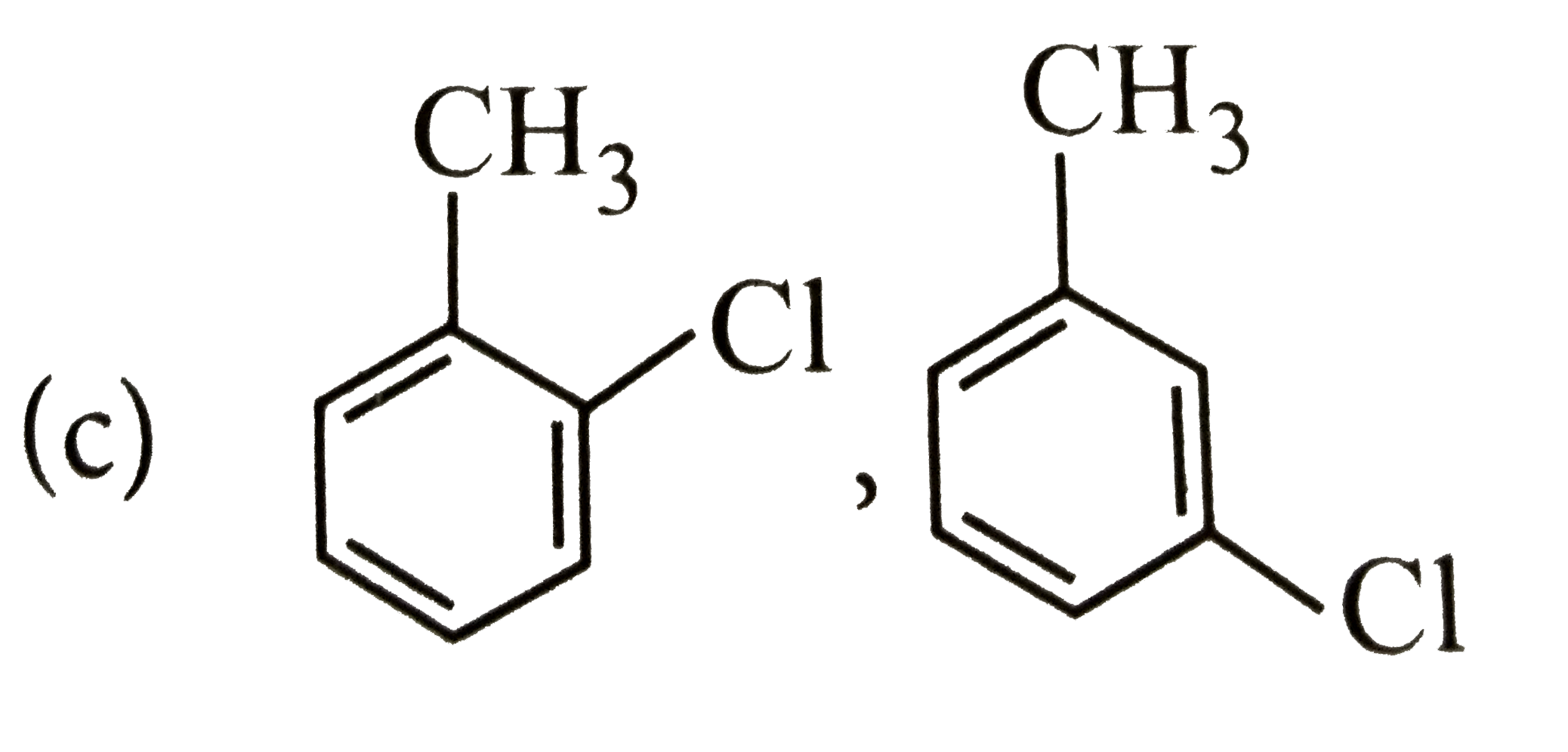
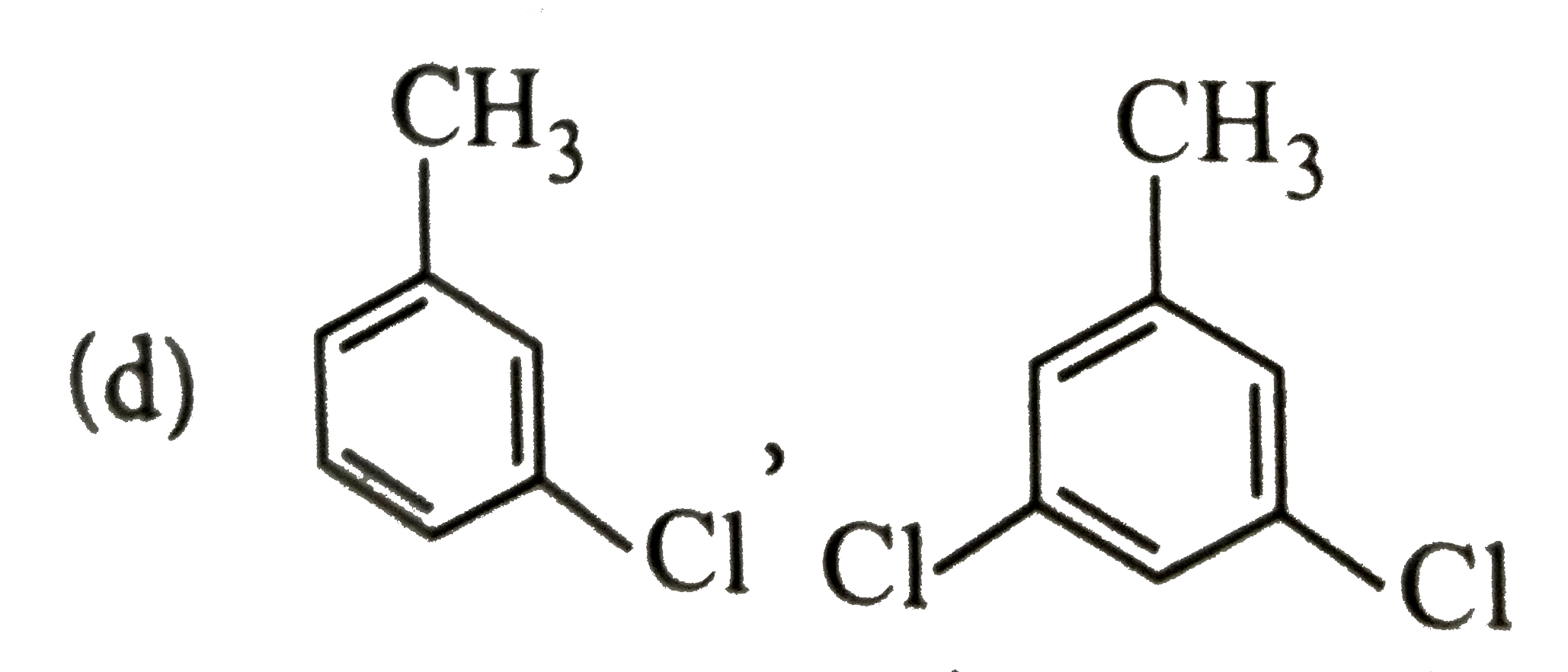
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise Physical properties|7 VideosHALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise Chemical reactions|68 VideosHALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise Nomenclature|6 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPOLYMERS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|9 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-HALOALKANES AND HALOARENES-Methods of preparation
- Halogen acids react with alcohols to form alkyl halides. The reaction ...
Text Solution
|
- Match the column I with column II and mark the appropriate choice.
Text Solution
|
- Which of the following compounds can yield only one monochlorinated pr...
Text Solution
|
- Bromination of methane in presence of sunlight is a
Text Solution
|
- A compound X with molecular formula C7H8 is treated with Cl2 in presen...
Text Solution
|
- Choose the correct option from the following :
Text Solution
|
- X and Y in the reaction are
Text Solution
|
- The reaction CH(2)=CH-CH(3)+HBr rarr CH(3)-overset(Br)overset(|)(C )HC...
Text Solution
|
- The negative part of the addendum (the molecule to be added ) adds on ...
Text Solution
|
- Which of the following reactions follows Markovnikov's rule?
Text Solution
|
- X in the reaction is
Text Solution
|
- Identify the products X and Y formed in the following reaction. CH3-...
Text Solution
|
- Methyl bromide reacts with AgF to give methyl fluoride and silver brom...
Text Solution
|