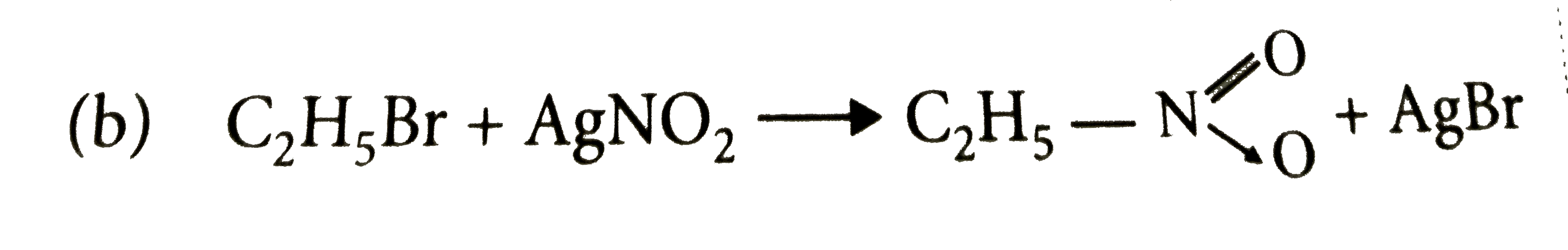A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise Higher order thinking skills|8 VideosHALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise Exemplar problems|24 VideosHALOALKANES AND HALOARENES
NCERT FINGERTIPS ENGLISH|Exercise Physical properties|7 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPOLYMERS
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|9 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-HALOALKANES AND HALOARENES-Chemical reactions
- Arrange the following compounds in order of their reactivity towards S...
Text Solution
|
- Which of the following haloalkanes is most reactive?
Text Solution
|
- Which of the following reactions does not take place?
Text Solution
|
- Consider the following bromides: The correct order of S(N^1) reac...
Text Solution
|
- Which of the following statement regarding S(N)1 reaction shown by alk...
Text Solution
|
- Consider the following reaction: C6H5-underset(H)underset(|)overset(...
Text Solution
|
- Which one of the following chlorohydrocarbons readily undergoes solvol...
Text Solution
|
- Which of the following is the most reactive towards nucleophilic subst...
Text Solution
|
- S(N^1) reaction is fastest in
Text Solution
|
- Which of the following alkyl halides is hydrolysed by S(N^(1)) mechani...
Text Solution
|
- Which of the following will give enantiomeric pair on reaction with wa...
Text Solution
|
- Which of the following is most reactive towards aqueous NaOH?
Text Solution
|
- In the following pairs of halogen compounds, which compounds undergoes...
Text Solution
|
- Which of the following haloalkanes reacts with aqueous KOH most easily...
Text Solution
|
- Which alkyl halide exhibits complete racemisation in S(N^1) reaction?
Text Solution
|
- The order of reactivity of various alkyl halides towards nucleophilic ...
Text Solution
|
- Alkyl halides are formed when thionyl chloride and are refluxed in pr...
Text Solution
|
- 2-Bromo-3,3- dimethylbutane on reaction with aqueous KOH yields X as t...
Text Solution
|
- Among the isomers of C5H11Cl, the one which is chiral is (i) 2,2-Dim...
Text Solution
|
- Ethylene dichloride and ethylidene chloride are isomeric compounds. Th...
Text Solution
|