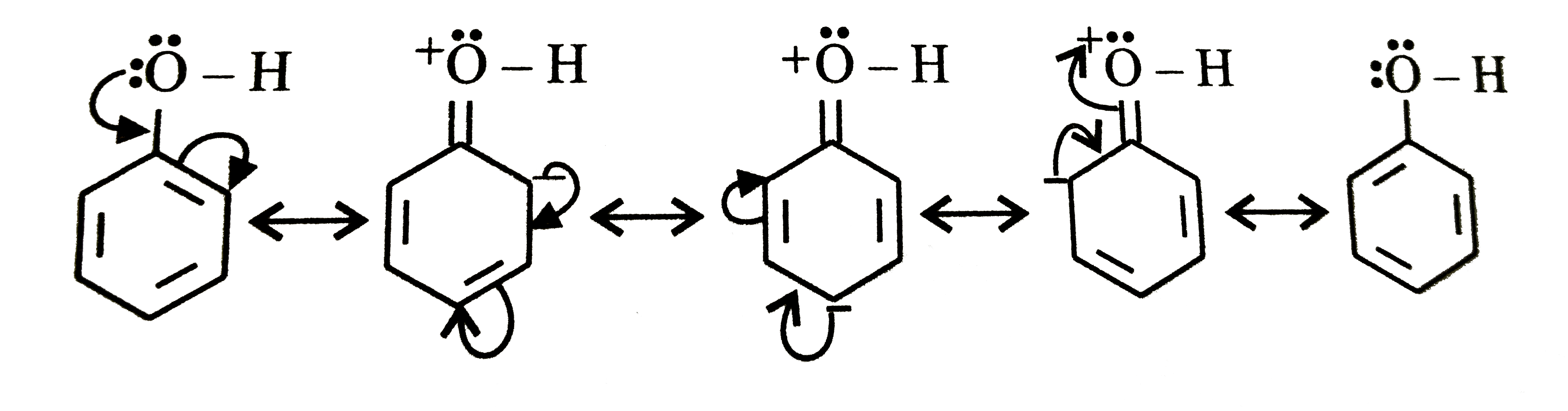
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ALCOHOLS,PHENOLS AND ETHERS-Assertion And Reason
- Out of phenol and benzene, which can be more easily nitrated ?
Text Solution
|
- Assertion : Catalytic reduction of butanal gives butanol. Reason: Al...
Text Solution
|
- Assertion (A) Addition reaction of water to but-1-ene in acidic medium...
Text Solution
|
- Assertion : Boiling point of ethanol is higher than that of propane. ...
Text Solution
|
- Assertion : Alcohols react both as nucleophiles and electrophiles. ...
Text Solution
|
- Assertio:pKa value of phenol is 10.0 while that of ethanol is 15.9 ...
Text Solution
|
- Assertion: Cresols are less acidic than phenol. Reason: Electron rele...
Text Solution
|
- Assertion: The relative ease of dehydration of alcohols following orde...
Text Solution
|
- Assertion : When the vapours of a primary, secondary or tertiary alcoh...
Text Solution
|
- Assertion: o-and p-nitrophenol can be separated by steam distillation....
Text Solution
|
- Assertion : Picric acid is a strong acid inspite of the absence of the...
Text Solution
|
- Assertion (A) Phenol forms 2, 4, 6-tribromophenol o treatement with Br...
Text Solution
|
- Assertion : Ethanol is obtained commercially by fermentation of molass...
Text Solution
|
- Assertion : When Alkyl aryl ethers react with excess of hydrogen halid...
Text Solution
|
- Assertion: Anisole undergoes eletctrophilic substitution at o-and p-po...
Text Solution
|