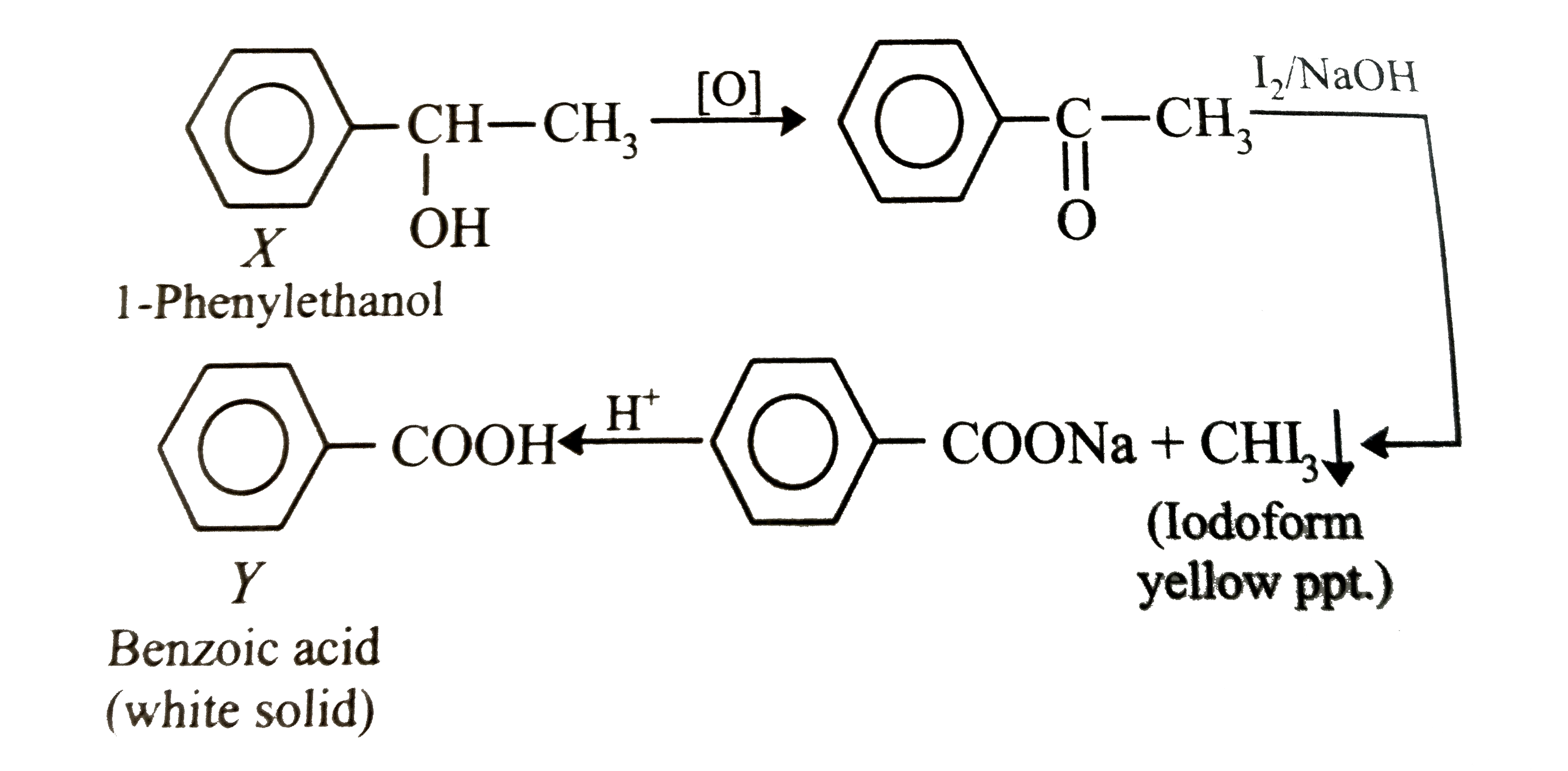Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise EXEMPLAR PROBLEMS|14 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise Classification|2 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|14 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ALCOHOLS,PHENOLS AND ETHERS-HOTS
- An alcohol (A) gives Lucas test within 5 min. 7.4 g of alcohol when tr...
Text Solution
|
- 2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of...
Text Solution
|
- 2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of...
Text Solution
|
- 2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of...
Text Solution
|
- 2.2 g of an alcohol (A) when treated with CH3 -Mgl liberates 560 mL of...
Text Solution
|
- A compund (a) of molecular formula C7H8O is insoluble in aqueous sodiu...
Text Solution
|
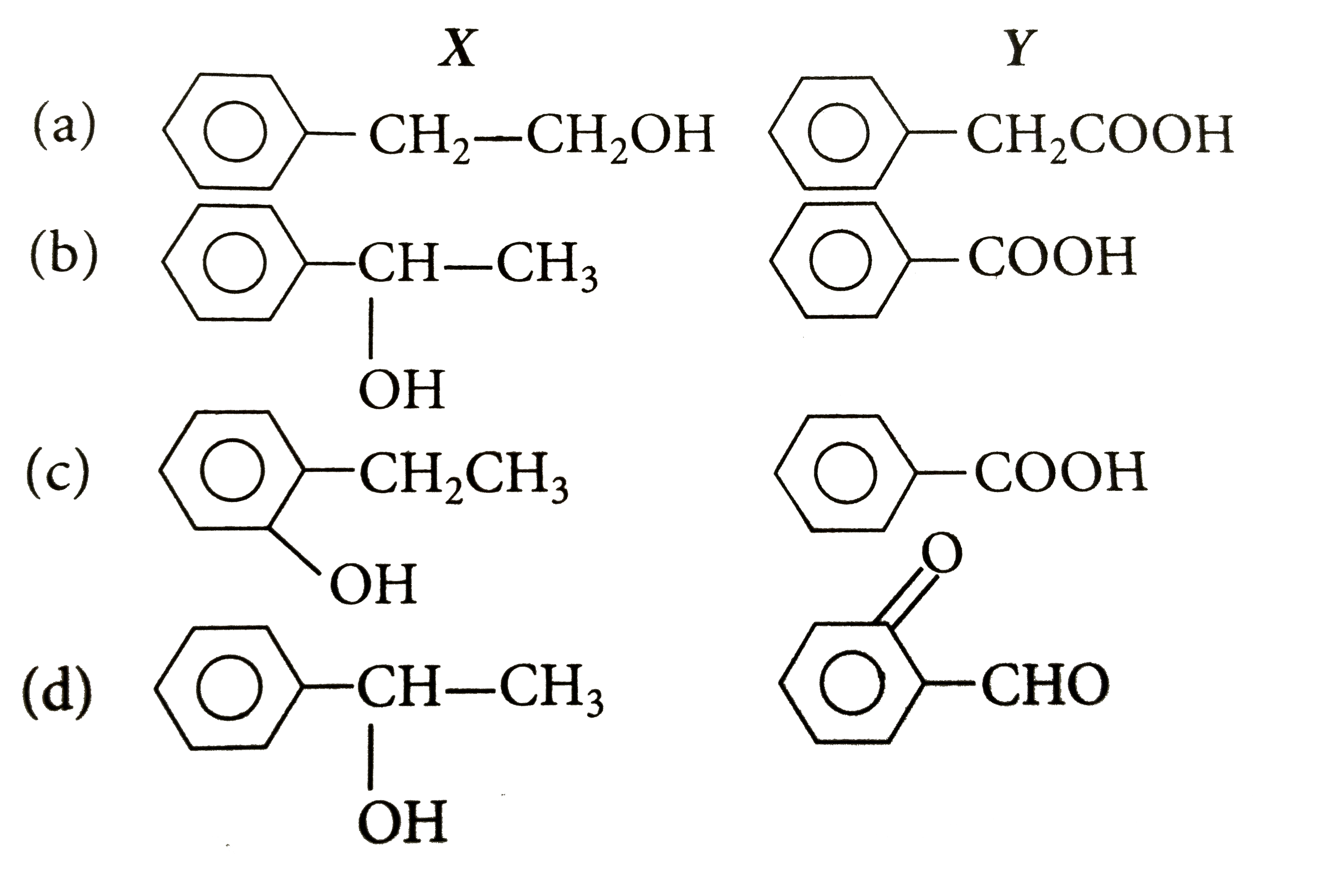
- A compund X(C8H10O) upon treatment with alkaline solution of iodine gi...
Text Solution
|