A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-ALCOHOLS,PHENOLS AND ETHERS-ASSERTION & REASON
- Assertion: The bond angle in alcohols is slighty less than the tetrah...
Text Solution
|
- Assertion : Catalytic reduction of butanal gives butanol. Reason: Al...
Text Solution
|
- Assertion (A) Addition reaction of water to but-1-ene in acidic medium...
Text Solution
|
- Assertion : Boiling point of ethanol is higher than that of propane. ...
Text Solution
|
- Assertion : Alcohols react both as nucleophiles and electrophiles. ...
Text Solution
|
- Assertio:pKa value of phenol is 10.0 while that of ethanol is 15.9 ...
Text Solution
|
- Assertion: Cresols are less acidic than phenol. Reason: Electron rele...
Text Solution
|
- Assertion: The relative ease of dehydration of alcohols following orde...
Text Solution
|
- Assertion : When the vapours of a primary, secondary or tertiary alcoh...
Text Solution
|
- Assertion: o-and p-nitrophenol can be separated by steam distillation....
Text Solution
|
- Assertion : Picric acid is a strong acid inspite of the absence of the...
Text Solution
|
- Assertion (A) Phenol forms 2, 4, 6-tribromophenol o treatement with Br...
Text Solution
|
- Assertion : Ethanol is obtained commercially by fermentation of molass...
Text Solution
|
- Assertion : When Alkyl aryl ethers react with excess of hydrogen halid...
Text Solution
|
- Assertion: Anisole undergoes eletctrophilic substitution at o-and p-po...
Text Solution
|
 in alcohols is slighty less than the tetrahedral angle.
in alcohols is slighty less than the tetrahedral angle. 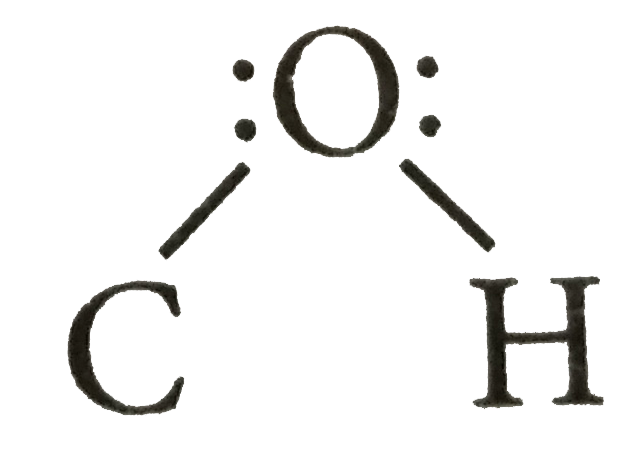 in alcohols is slightly less than the retrahedral angle due to the repulsion between the unshared electron pairs of oxygen.
in alcohols is slightly less than the retrahedral angle due to the repulsion between the unshared electron pairs of oxygen.