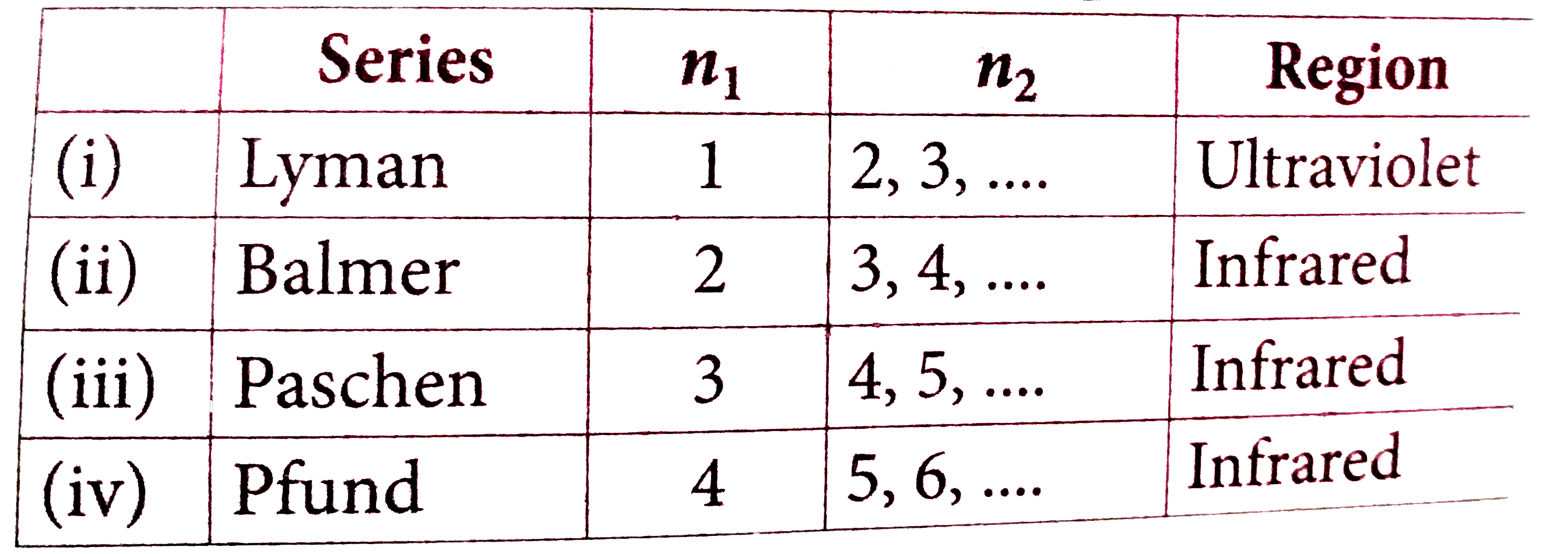A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise Towards Quantum Mechanical Model Of The Town|10 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise Quantum Mechanical Model Of Atom|38 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise Developments Leading To The Bohr'S Model Of Atom|14 VideosSTATES OF MATTER
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-STRUCTURE OF ATOM -Bohr'S Model For Hydrogen Atom
- The electron in Bohr's model of hydrogen atom is pictured as revolving...
Text Solution
|
- Given below are the spectral lines for an atom of hydrogen. Mark the l...
Text Solution
|
- What is the maximum number of emission lines when the excited electro...
Text Solution
|
- What is the maximum number of emission lines obtained when the excited...
Text Solution
|
- What is the colour corresponding to the wavelength of light emitted wh...
Text Solution
|
- Which one of the series of hydrogen spectrum is in the visible region ...
Text Solution
|
- An electron in excited hydrogen atom falls from fifth energy level to ...
Text Solution
|
- The third line of the Balmer series, in the emission spectrum of the h...
Text Solution
|
- The frequency of radiation absorbed or emitted when transition occurs ...
Text Solution
|
- The angular momentum of an electron in a given stationary state can be...
Text Solution
|
- According to Bohr's theory the angular momentum of an electron in 5th ...
Text Solution
|
- The radius of the stationary state which is also called Bohr radius is...
Text Solution
|
- If the radius of first Bohr orbit be a0, then the radius of the third ...
Text Solution
|
- What does the negative electronic energy (negative sign for all values...
Text Solution
|
- The energy of the electron in a hydrogen atom has a negative sign for ...
Text Solution
|
- If the ionization energy for the hydrogen atom is 13.6 eV , the energy...
Text Solution
|
- Bohr's theory can also be applied to the ions like
Text Solution
|
- According to Bohr's theory, the electronic energy of H-atom in Bohr's ...
Text Solution
|
- The Bohr's energy of a stationary state of hydrogen atom is given as E...
Text Solution
|
- Which of the following is not correctly matched?
Text Solution
|