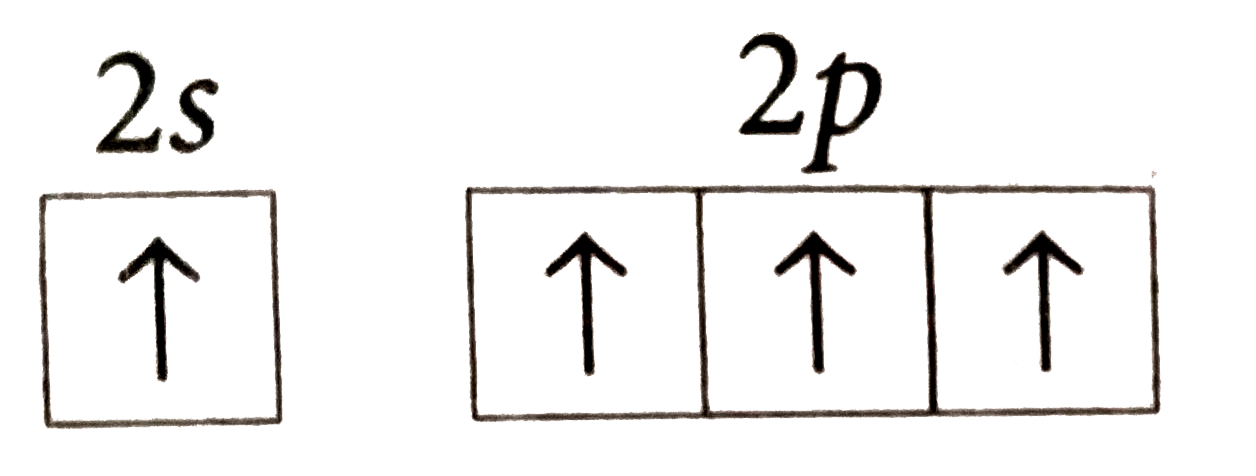
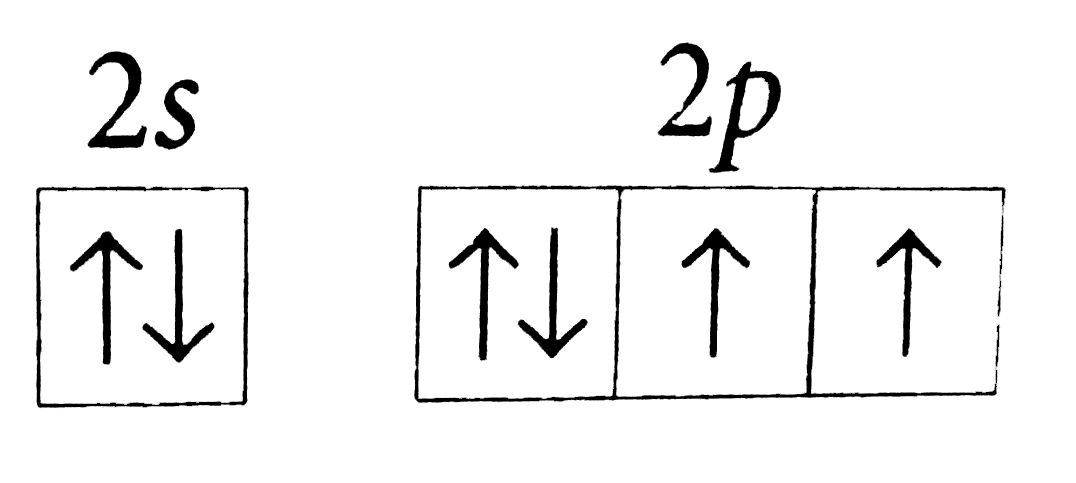
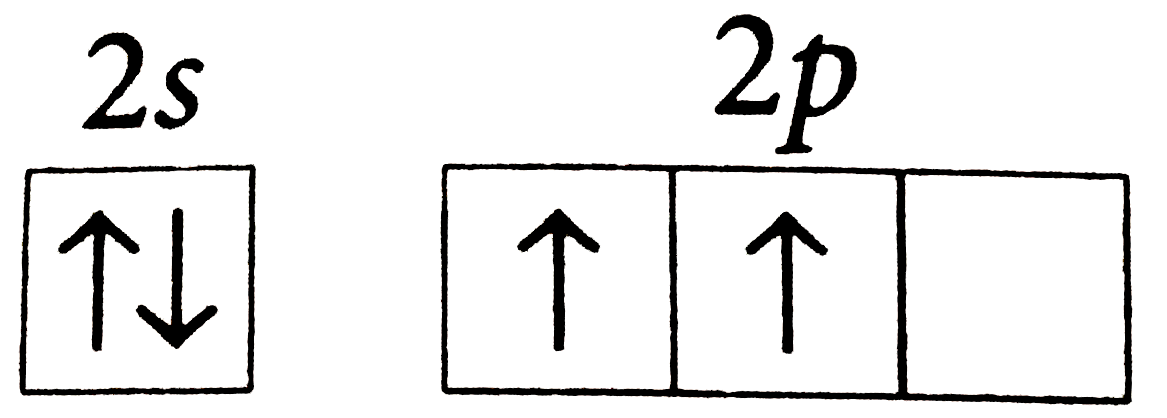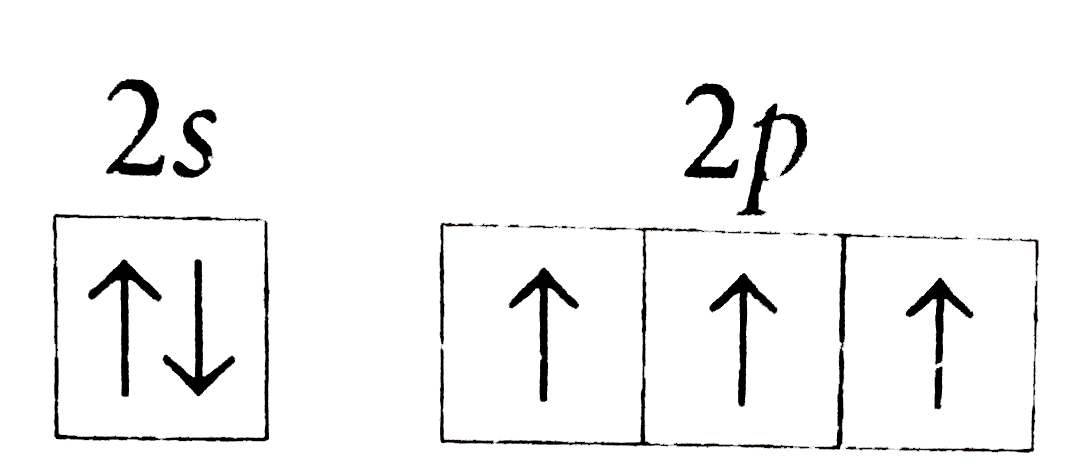A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise HIGHER ORDER THINKING SKILLS|11 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise NCERT EXEMPLAR PROBLEMS|16 VideosSTATES OF MATTER
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-STRUCTURE OF ATOM -Assertion And Reason
- The orbital diagram in which the Aufbau principle is violated is
Text Solution
|
- Assertion : The characteristics of cathode rays do not depend upon the...
Text Solution
|
- Assertion : X-rays are used to study the interior of objects . Rea...
Text Solution
|
- Assertion : In Rutherford's alpha-particle scattering experiment, most...
Text Solution
|
- Assertion : All the isotopes of a given element show same chemical beh...
Text Solution
|
- Assertion : In electromagnetic spectrum, the small portion around 10^(...
Text Solution
|
- Assertion : When an iron rod is heated in a furnace, the radiation emi...
Text Solution
|
- Assertion : The number of electrons ejected from a metal surface depen...
Text Solution
|
- Assertion : Elements like Rb, Cs, Tl, In, Ga and Sc were discovered wh...
Text Solution
|
- Assertion : In hydrogen and hydrogen like species, orbital energy depe...
Text Solution
|
- Assertion : According to de Broglie, the wavelengths associated with e...
Text Solution
|
- Assertion : For l = 2, m(l) can be -2, -1, 0, +1 and +2. Reason : Fo...
Text Solution
|
- Assertion : Orbit and orbital are synonymous. Reason : A circular pa...
Text Solution
|
- Assertion : 3d(z^(2)) orbital is spherically symmetrical. Reason : T...
Text Solution
|
- Assertion : The maximum number of electrons in the shell with principl...
Text Solution
|
- Assertion : The outer electronic configuration of Cr and Cu are 3d^...
Text Solution
|