A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON|15 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise Discovery Of Sub-Atomic Particles|5 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS ENGLISH|Exercise HIGHER ORDER THINKING SKILLS|11 VideosSTATES OF MATTER
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-STRUCTURE OF ATOM -NCERT EXEMPLAR PROBLEMS
- Which of the following conclusions could not be derived from Rutherfor...
Text Solution
|
- Which of the following options does not represent ground state electr...
Text Solution
|
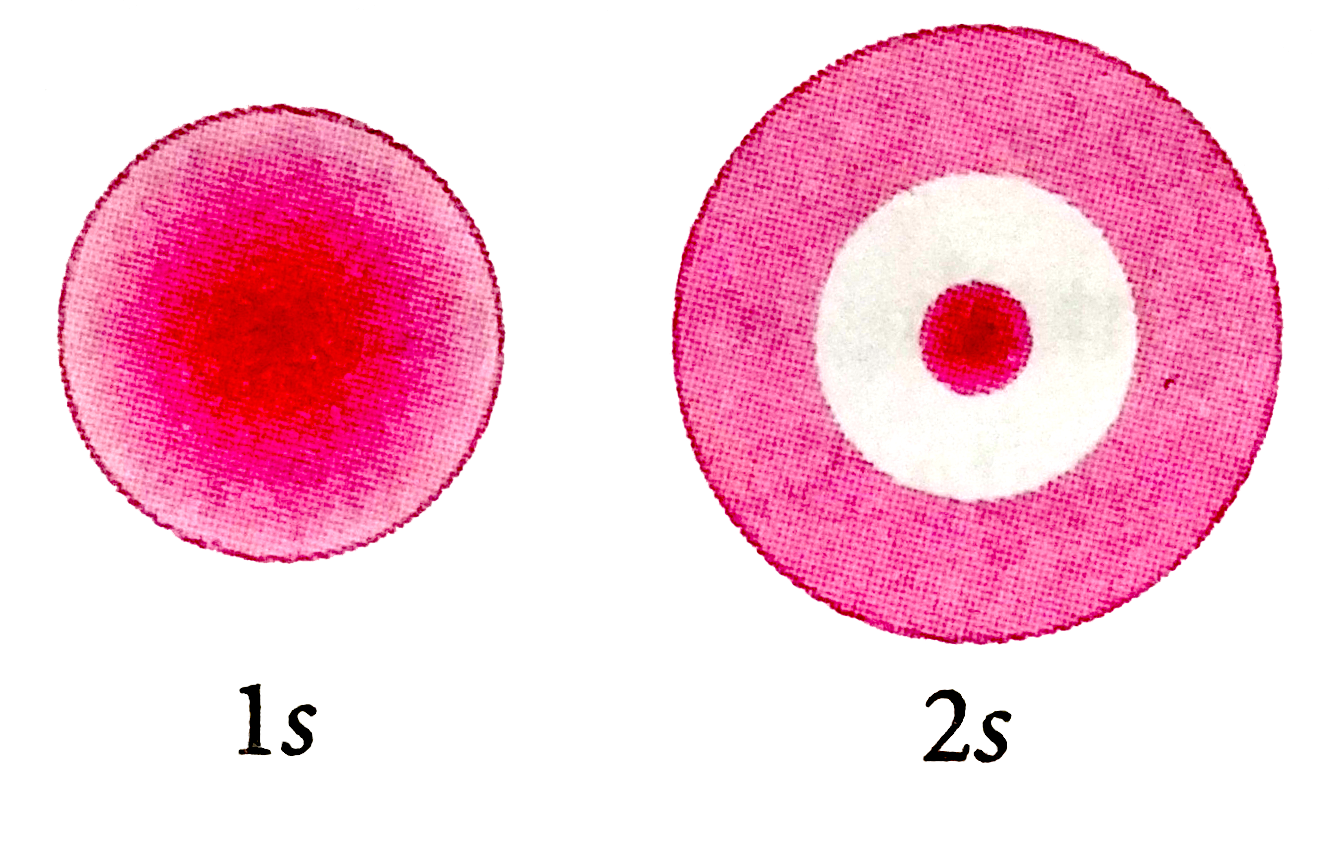
- The probability density plots of 1s and 2s atomic orbitals are given i...
Text Solution
|
- Which of the following statement is not correct about the charactersti...
Text Solution
|
- Which of the following statements about the electron is incorrect?
Text Solution
|
- Which of the following properties of atom could be explained correctl...
Text Solution
|
- Two atoms are said to be isobars is
Text Solution
|
- The number of radial nodes for 3p orbital is......
Text Solution
|
- Number of angular nodes for 4d orbital is.........
Text Solution
|
- Which of the following is responsible to rule out the existence of def...
Text Solution
|
- Total number of orbitals associated with third shell will be.....
Text Solution
|
- Orbital angular momentum depends on .........
Text Solution
|
- Chlorine exists in two isotopic forms Cl-37 and Cl-35 but its atomic m...
Text Solution
|
- The pair of ions having same electronic configuration is.
Text Solution
|
- For the electrons of oxygen atom, which of the following statements c...
Text Solution
|
- If travelling at same speeds, which of the following matter waves have...
Text Solution
|