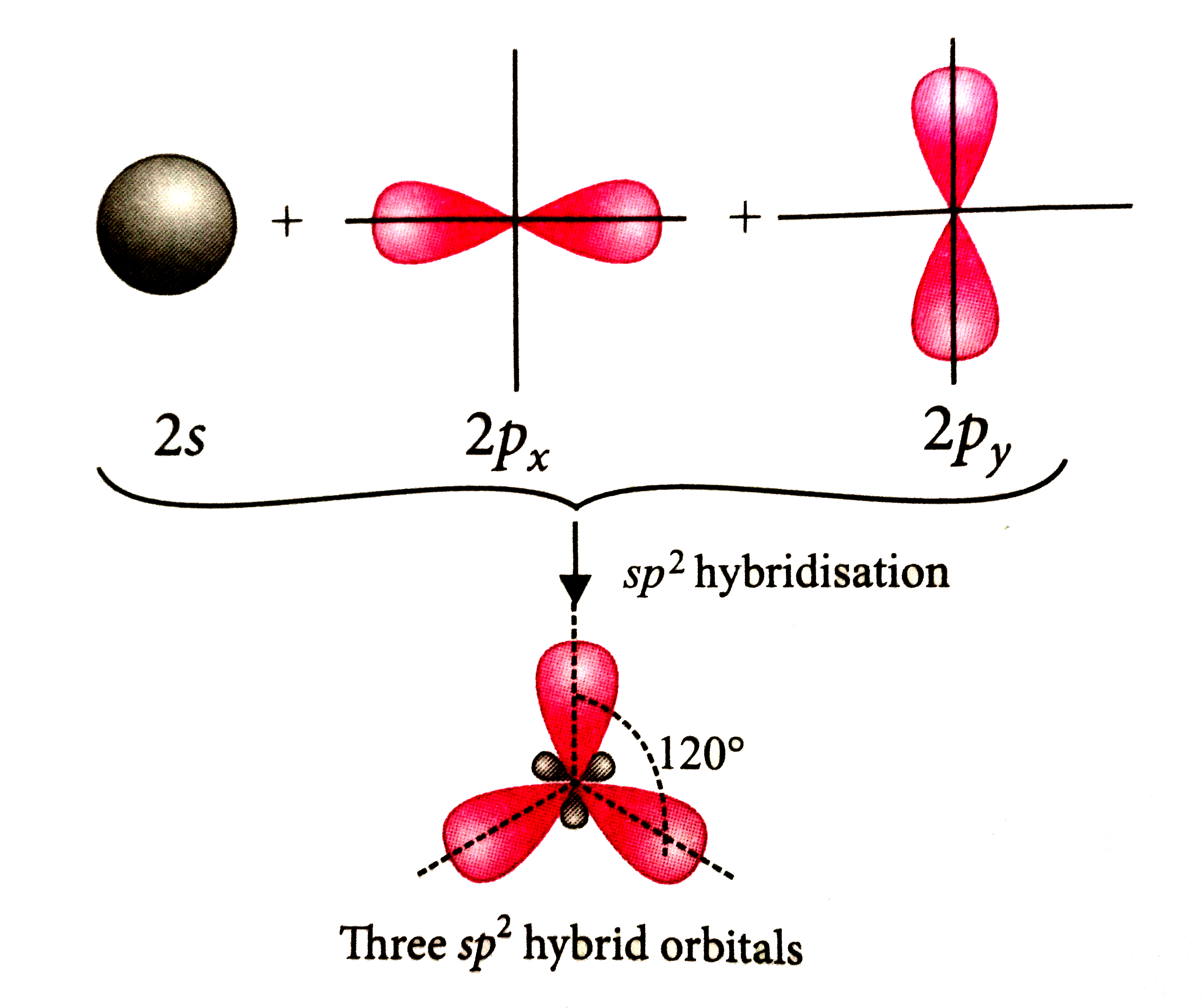
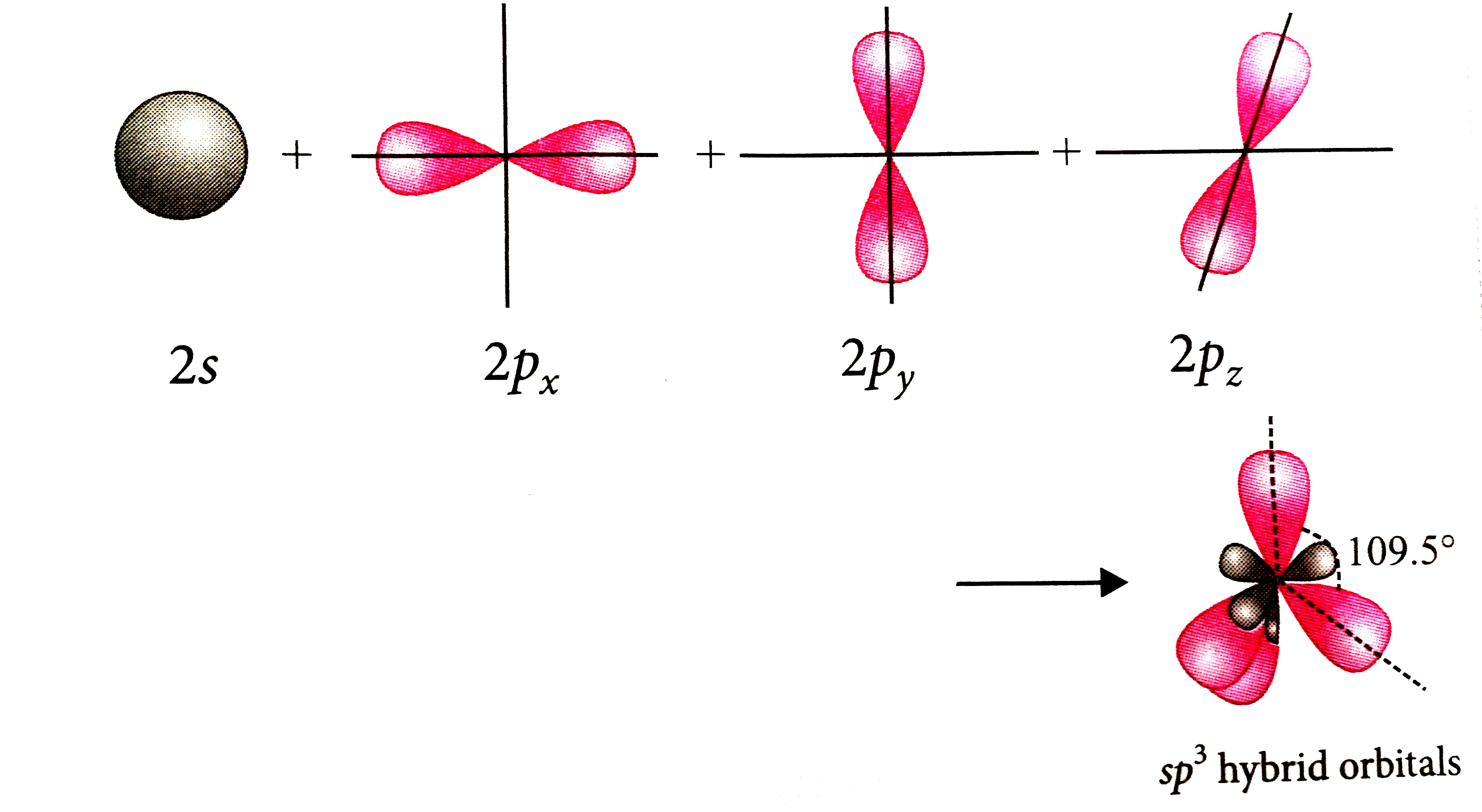A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS ENGLISH|Exercise HIGHER ORDER THINKING SKILLS|10 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
NCERT FINGERTIPS ENGLISH|Exercise EXEMPLAR PROBLEMS|14 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-CHEMICAL BONDING & MOLECULAR STRUCTURE-Assertion And Reason
- Which of the following statements is true about hybridisation ?
Text Solution
|
- Assertion : In the formation of a molecule , only the outer shell ele...
Text Solution
|
- Assertion : In the formation of water molecule, both hydrogen and oxyg...
Text Solution
|
- Assertion : In Liwis structures of NF(3) and CO(3)^(2-), nitrogen and...
Text Solution
|
- Assertion : PF(5),SF(6)and H(2)SO(4) are the examples of expanded octe...
Text Solution
|
- Assertion : Octet rule is based upon the chemical inertness of noble g...
Text Solution
|
- Assertion : Sodium chloride (NaCl) is a stable ionic solid. Reason ...
Text Solution
|
- Assertion : F(2)and O(2)^(2-)have bond order 1 while N(2),CO and NO^(+...
Text Solution
|
- Assertion : The experimentally determined carbon to oxygen bond length...
Text Solution
|
- Assertion : The dipole moment in case of BeF(2) is zero. Reason : T...
Text Solution
|
- Assertion : Dipole moment of NH(3) is greater than that of NF(3). Re...
Text Solution
|
- Assertion : Among alkaline earth metals, Be predominantly forms covale...
Text Solution
|
- Assertion : In NH(3), N "is " sp^(3) hybridised but bond angle is 107^...
Text Solution
|
- Ionic bonds are non-directional while covalent bonds are directional.
Text Solution
|
- Assertion : O(2) molecule is diamagnetic while C(2) molecule is parama...
Text Solution
|
- Assertion : Boiling point of p-nitrophenol is greater than that of o-n...
Text Solution
|