Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THERMODYNAMICS-Assertion And Reason
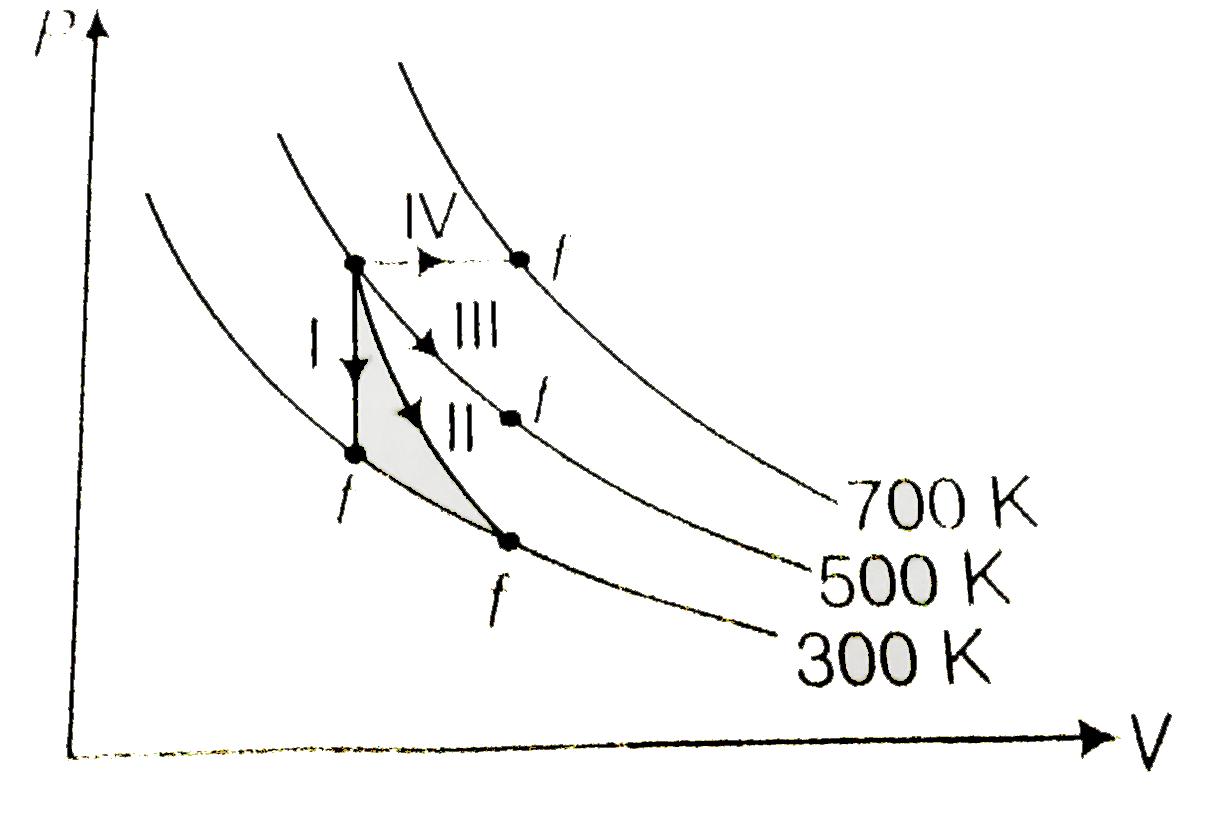
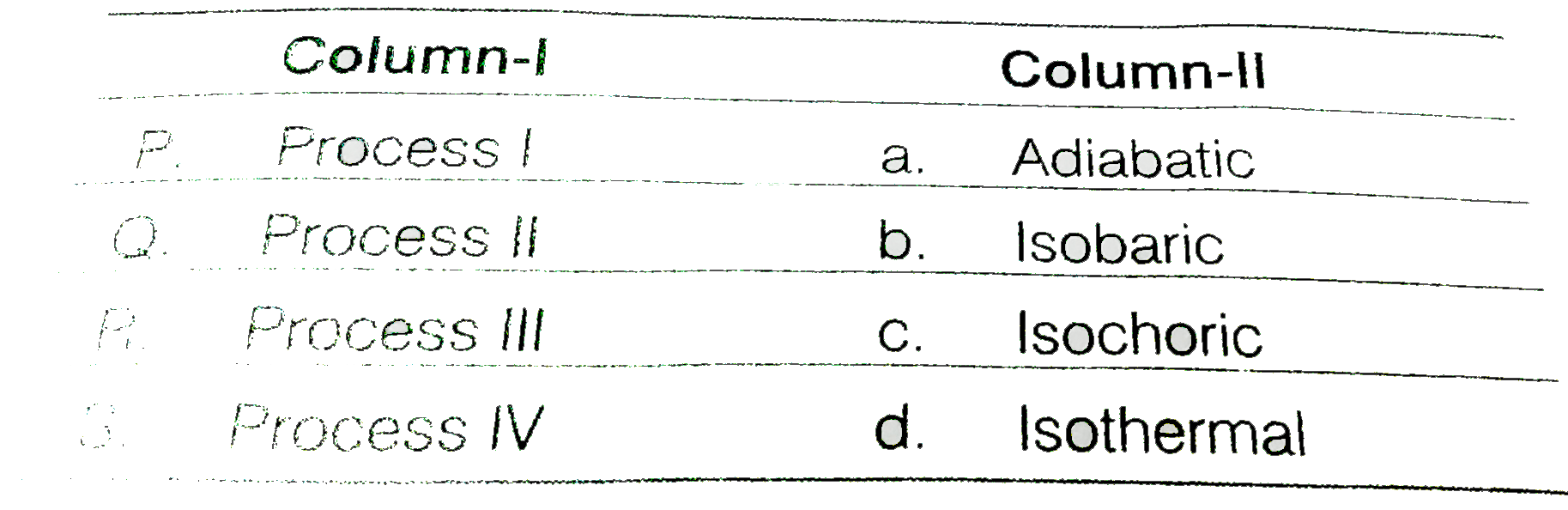
- Thermodynamic processes are indicated in the following diagram M...
Text Solution
|
- Assertion : The presence of reactants in a closed vessel made of condu...
Text Solution
|
- Assertion : In adiabatic system, DeltaU=w(ad.) Reason : In adiabatic...
Text Solution
|
- Assertion (A): Internal energy change in a cyclic process is zero. ...
Text Solution
|
- Assertion : Work done during free expansion of an ideal gas whether re...
Text Solution
|
- Assertion : The difference between DeltaH and DeltaU is not significan...
Text Solution
|
- Assertion : For the change, H(2)O((l))rarr H(2)O((s)),DeltaH=DeltaU. ...
Text Solution
|
- Assertion : DeltaH for an exothermic reaction is negative nad for an e...
Text Solution
|
- Assertion : The enthalpy change for the reaction CaO((s))+CO(2(g)) rar...
Text Solution
|
- Assertion : The solubility of most salts in water increases with rise ...
Text Solution
|
- Assertion : Heat of neutralisation of HNO(3) and NaOH is same as that ...
Text Solution
|
- Assertion : Heat added to a system at lower temperature causes greate...
Text Solution
|
- Assertion : In the proces, H(2(g)) rarr 2H((g)).entropy increases. R...
Text Solution
|
- Assertion : An exothermic process which is non-spontaneous at high tem...
Text Solution
|
- Assertion : If both DeltaH^(@) and DeltaS^(@) are positive then reacti...
Text Solution
|
- Assertion : Third law of thermodynamics is confined to pure crystallin...
Text Solution
|